The Challenge of Vulnerable Plaque Detection in the Cardiac Catheterization Laboratory †
Summary
Introduction
- A.
- Thin-cap fibro-atheroma (TCFA): in about 65% of all symptomatic coronary thrombotic events, rupture of an inflamed TCFA was evident. The major components of such TCFA are: an atheromatous core (usually >40% of the entire plaque), a thin fibrous cap with macrophage and lymphocyte infiltration and decreased smooth muscle cell content, and expansive remodeling [2].
- B.
- Erosion: in about 30% of all events, the endothelium overlying the plaque was found injured at the place where a thrombus has formed. Usually, these plaques are rich in proteoglycans [3].
- C.
- A calcified nodule: in 5% of all events, thrombosis covering a calcified nodule suggests that the plaque appeared to be heavily calcified with a calcified nodule projecting into the lumen [4].
Angiography
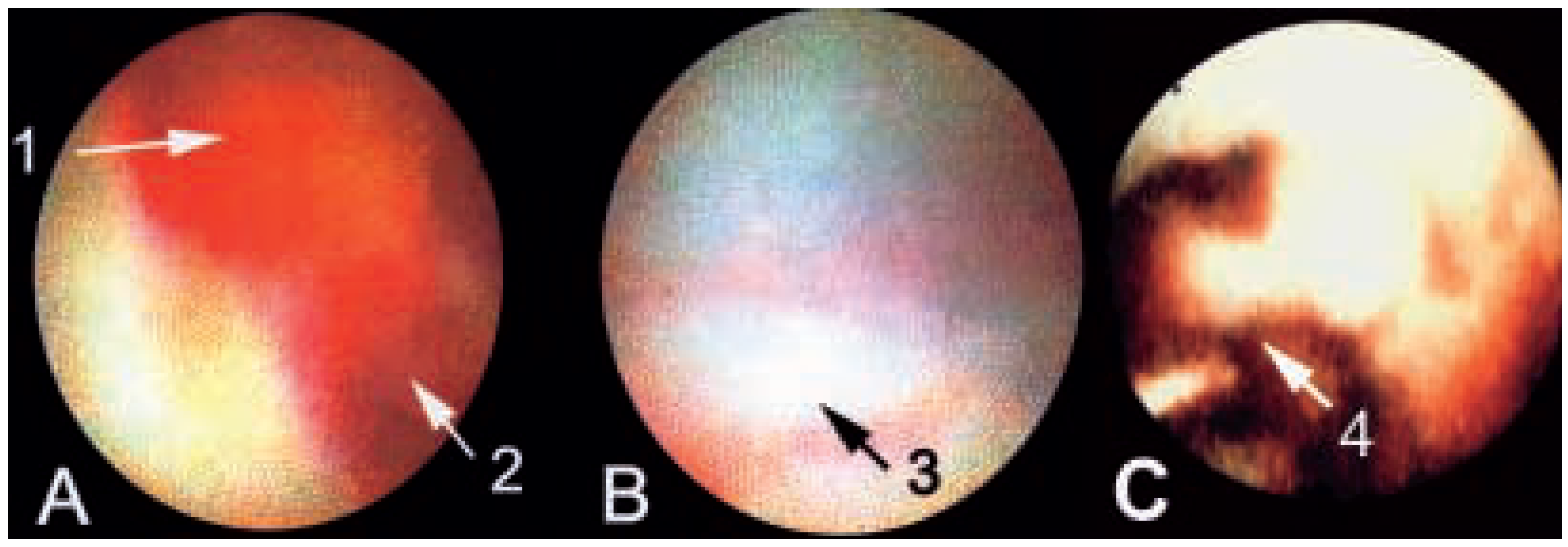
Angioscopy
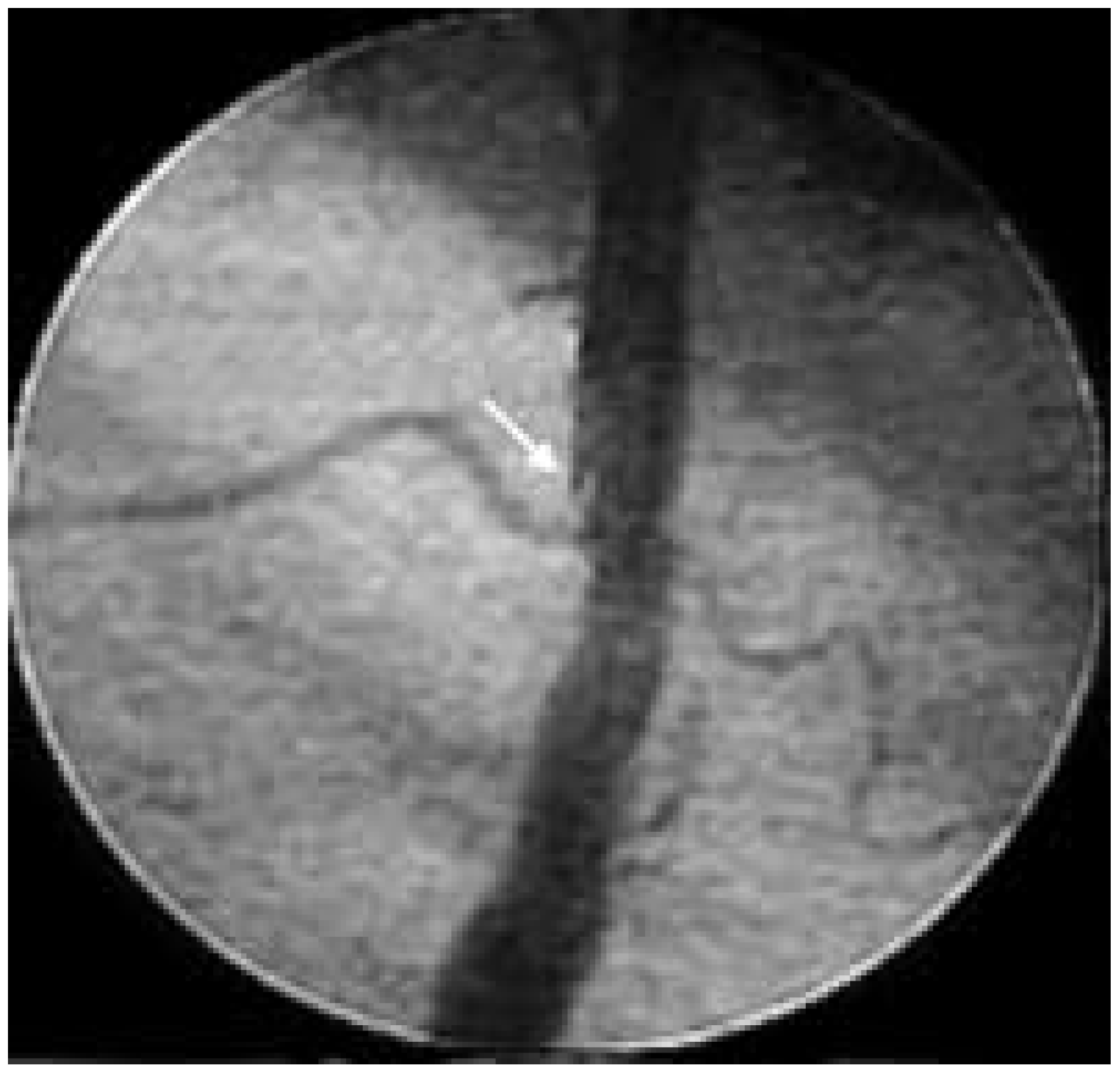
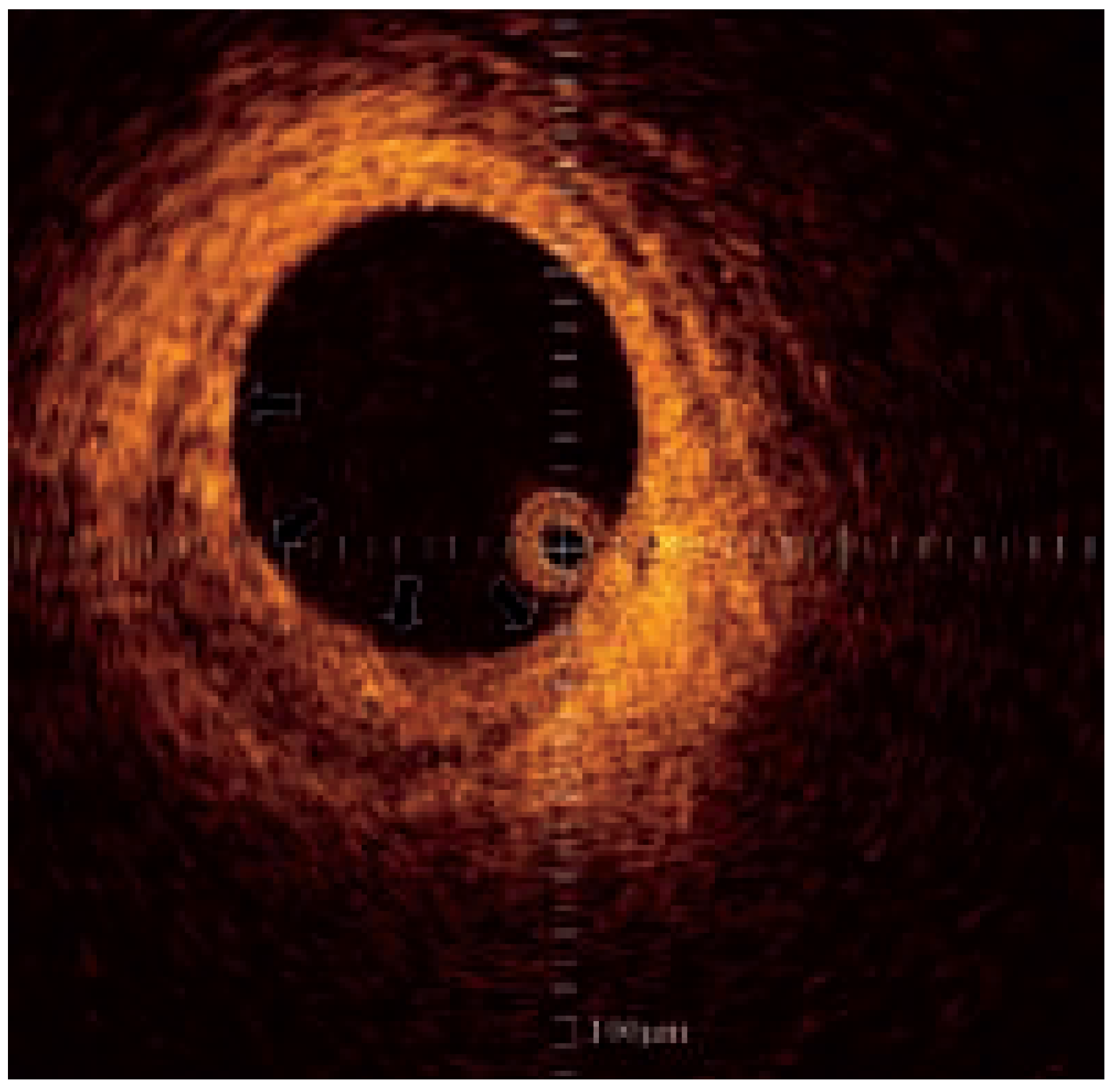
Intra-vascular ultrasound
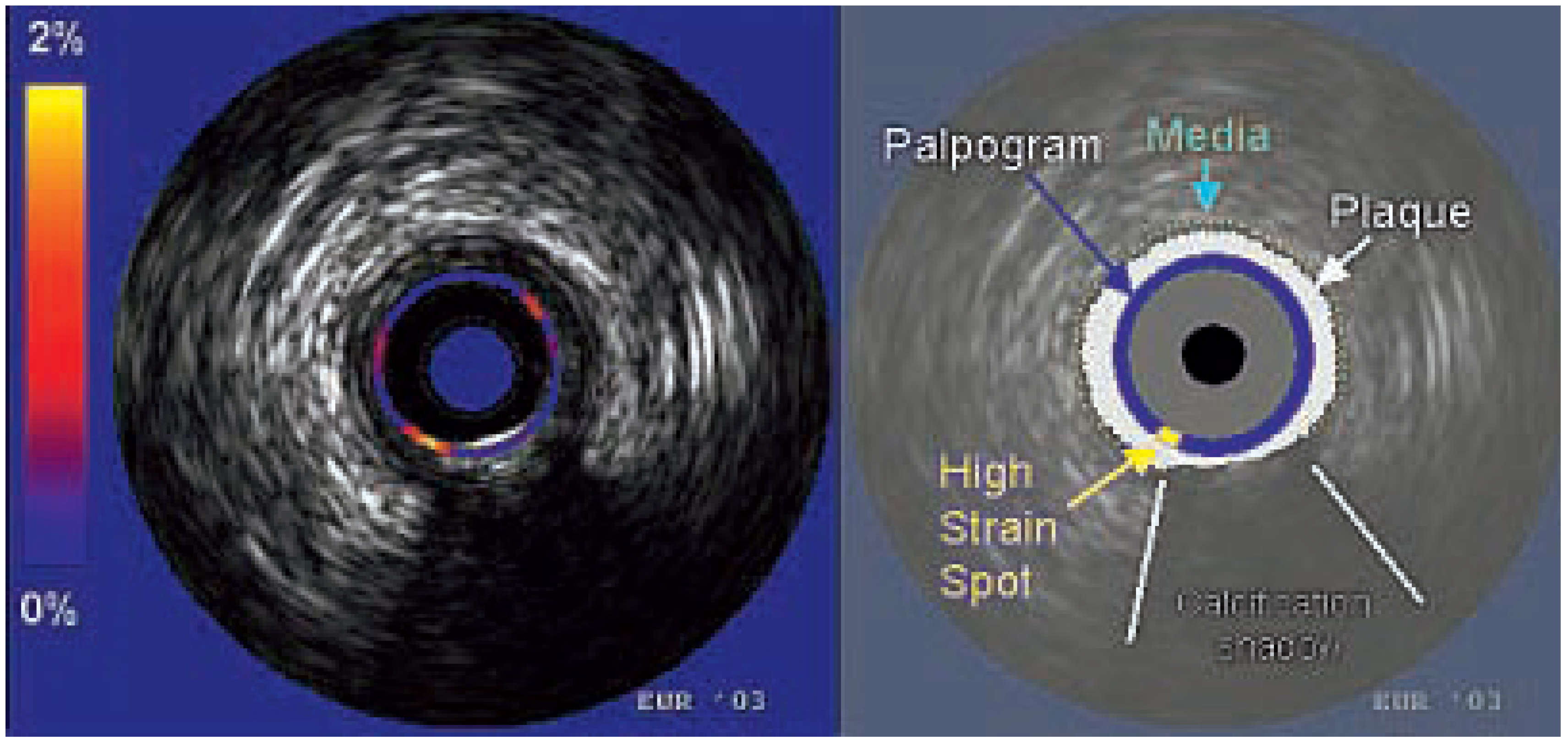
Intra-vascular elastography/palpography
Thermography
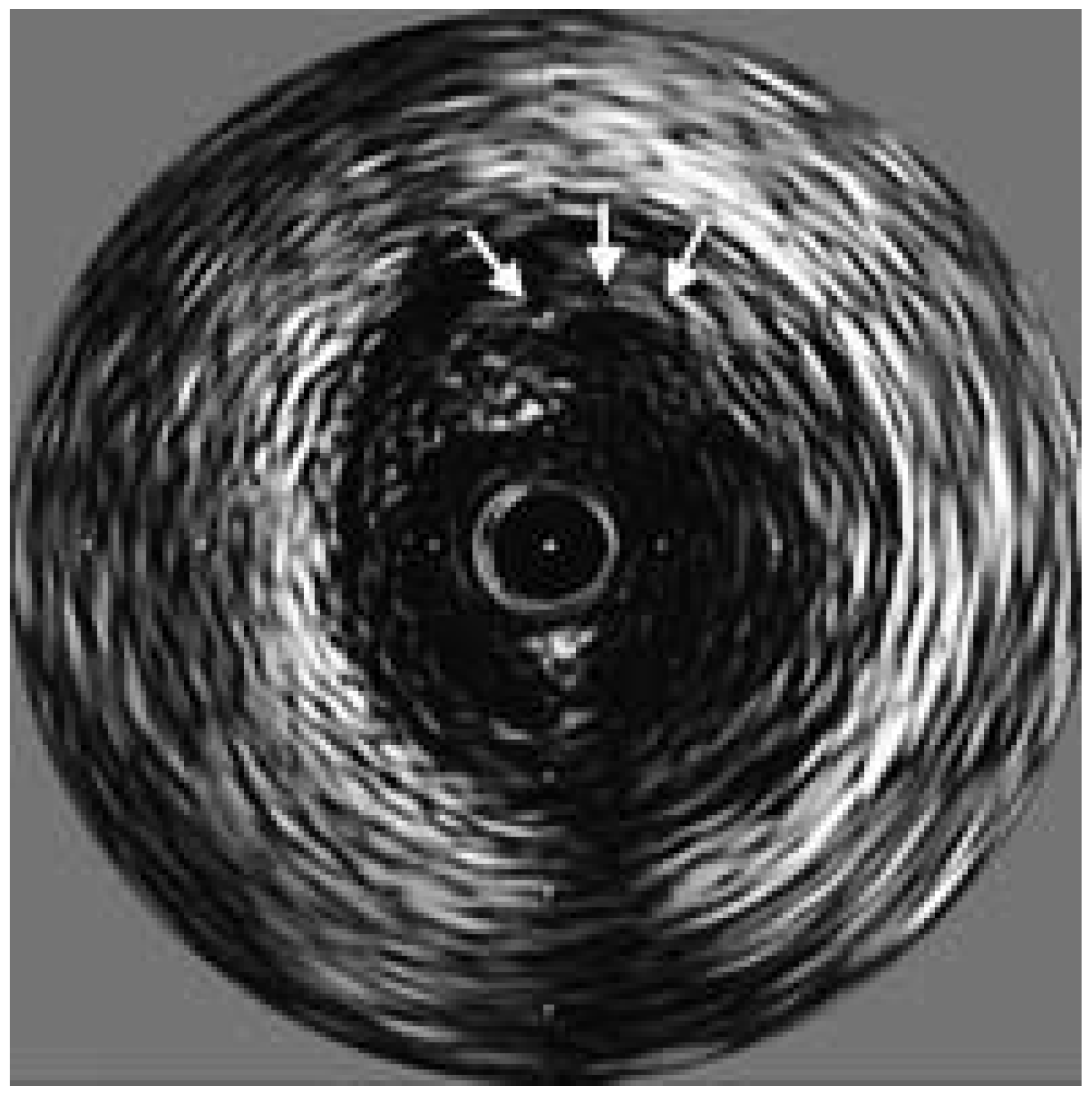
Optical coherence tomography
Spectroscopy
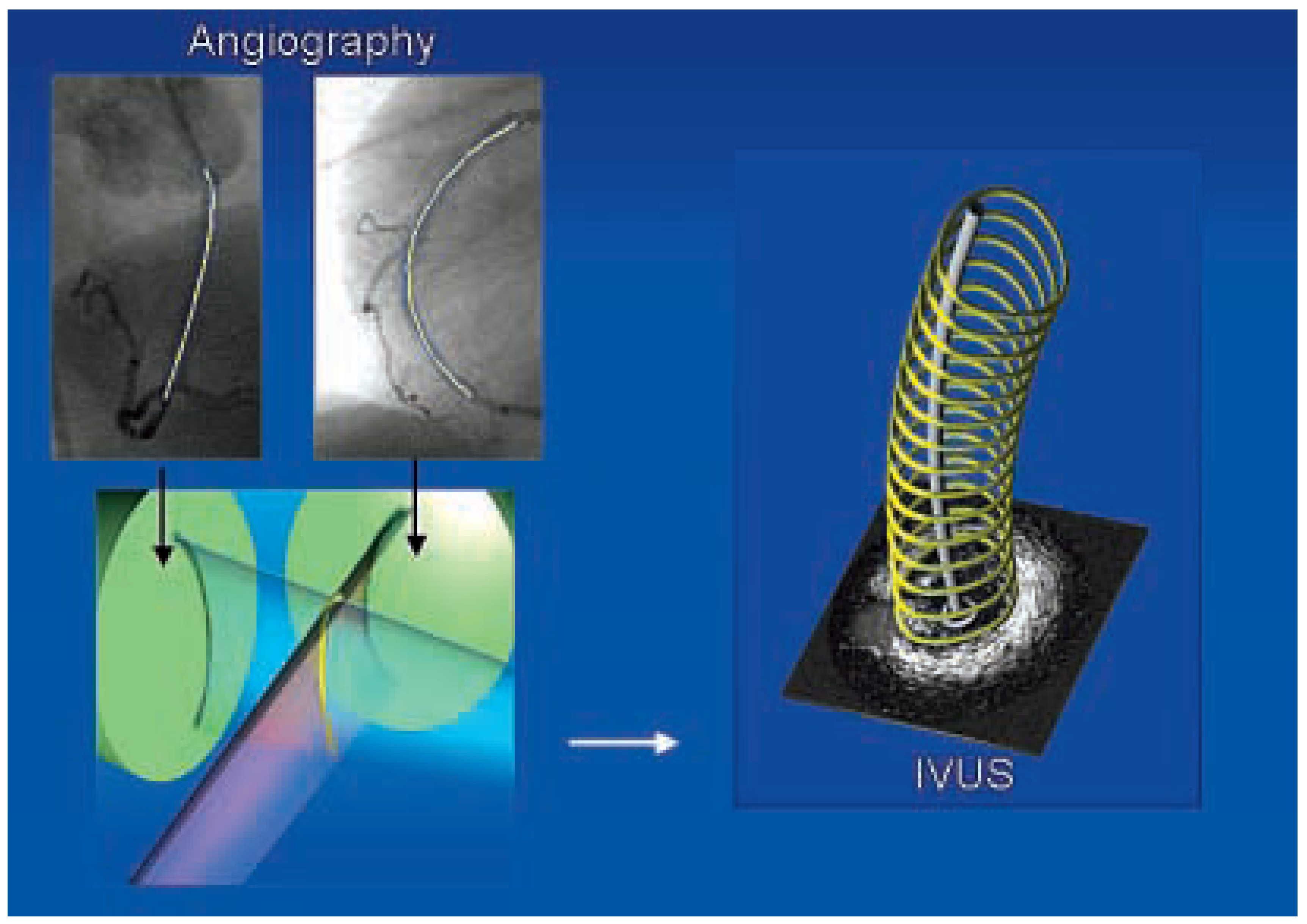
ANGUS and shear stress
Conclusions
Funding
Conflicts of Interest
References
- Schaar, J.A.; Muller, J.E.; Falk, E.; Virmani, R.; Fuster, V.; Serruys, P.W.; et al. Terminology for high-risk and vulnerable coronary artery plaques. Eur Heart J 2004, 25, 1077–1082. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Farb, A.; Gold, H.K.; Yuan, J.; Narula, J.; et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol 2001, 16, 285–292. [Google Scholar] [CrossRef]
- van der Wal, A.C.; Becker, A.E.; van der Loos, C.M. Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Demetriou, D.; Grines, C.L.; Pica, M.; Shoukfeh, M.; O’Neill, W.W. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med 2000, 343, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Frink, R.J. Chronic ulcerated plaques: new insights into the pathogenesis of acute coronary disease. J Invasive Cardio 1004, 6, 173–185. [Google Scholar]
- Little, W.C.; Constantinescu, M.; Applegate, R.J.; Kutcher, M.A.; Burrows, M.T.; Kahl, F.R.; et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 1988, 78, 1157–1166. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Tannenbaum, M.A.; Alexopoulos, D.; HjemdahlMonsen, C.E.; Leavy, J.; Weiss, M.; et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1988, 12, 56–62. [Google Scholar]
- Ojio, S.; Takatsu, H.; Tanaka, T.; Ueno, K.; Yokoya, K.; Matsubara, T.; et al. Considerable time from the onset of plaque rupture and/or thrombi until the onset of acute myocardial infarction in humans: coronary angiographic findings within 1 week before the onset of infarction. Circulation 2000, 102, 2063–2069. [Google Scholar] [CrossRef]
- Asakura, M.; Ueda, Y.; Yamaguchi, O.; Adachi, T.; Hirayama, A.; Hori, M.; et al. Extensive development of vulnerable plaques as a pan-coronary process in patients with myocardial infarction: an angioscopic study. J Am Coll Cardiol 2001, 37, 1284–1288. [Google Scholar] [CrossRef]
- Uchida, Y.; Nakamura, F.; Tomaru, T.; Morita, T.; Oshima, T.; Sasaki, T.; et al. Prediction of acute coronary syndromes by percutaneous coronary angioscopy in patients with stable angina. Am Heart J 1995, 130, 195–203. [Google Scholar] [CrossRef]
- Di Mario, C.; The, S.H.; Madretsma, S.; van Sluyten, R.J.; Wilson, R.A.; Bom, N.; et al. Detection and characterization of vascular lesions by intravascular ultrasound: an in vitro study correlated with histology. J Am Soc Echocardiogr 1992, 5, 135–146. [Google Scholar] [CrossRef]
- Friedrich, G.J.; Moes, N.Y.; Muhlberger, V.A.; Gabl, C.; Mikuz, G.; Hausmann, D.; et al. Detection of intralesional calcium by intracoronary ultrasound depends on the histologic pattern. Am Heart J 1994, 128, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Potkin, B.N.; Bartorelli, A.L.; Gessert, J.M.; Neville, R.F.; Almagor, Y.; Roberts, W.C.; et al. Coronary artery imaging with intravascular high-frequency ultrasound. Circulation 1990, 81, 1575–1585. [Google Scholar] [CrossRef]
- Hiro, T.; Leung, C.Y.; Russo, R.J.; Karimi, H.; Farvid, A.R.; Tobis, J.M. Variability of a three-layered appearance in intravascular ultrasound coronary images: a comparison of morphometric measurements with four intravascular ultrasound systems. Am J Card Imaging 1996, 10, 219–227. [Google Scholar]
- Ge, J.; Chirillo, F.; Schwedtmann, J.; Gorge, G.; Haude, M.; Baumgart, D.; et al. Screening of ruptured plaques in patients with coronary artery disease by intravascular ultrasound. Heart 1999, 81, 621–627. [Google Scholar] [CrossRef]
- Riouful, G.; Finet, G.; Ginon, I.; Andre-Fouet, X.; Rossi, R.; Vialle, E.; et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation 2002, 106, 804–808. [Google Scholar] [CrossRef]
- Fujii, K.; Kobayashi, Y.; Mintz, G.S.; Takebayashi, H.; Dangas, G.; Moussa, I.; et al. Intravascular ultrasound assessment of ulcerated ruptured plaques: a comparison of culprit and nonculprit lesions of patients with acute coronary syndromes and lesions in patients without acute coronary syndromes. Circulation 2003, 108, 2473–2478. [Google Scholar] [CrossRef]
- Yamagishi, M.; Terashima, M.; Awano, K.; Kijima, M.; Nakatani, S.; Daikoku, S.; et al. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol 2000, 35, 106–111. [Google Scholar] [CrossRef] [PubMed]
- von Birgelen, C.; Klinkhart, W.; Mintz, G.S.; Papatheodorou, A.; Hermann, J.; Baumgart, D.; et al. Plaque distribution and vascular remodeling of ruptured and non-ruptured coronary plaques in the same vessel: an intravascular ultrasound study in vivo. J Am Coll Cardiol 2001, 37, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Kuban, B.D.; Obuchowski, N.; Vince, D.G. Assessing spectral algorithms to predict atherosclerotic plaque composition with normalized and raw intravascular ultrasound data. Ultrasound Med Biol 2001, 10, 1319–1331. [Google Scholar] [CrossRef]
- Landini, L.; Sarnelli, R.; Picano, E.; Salvadori, M. Evaluation of frequency dependence of backscatter coefficient in normal and atherosclerotic aortic walls. Ultrasound Med Biol 1986, 5, 397–401. [Google Scholar] [CrossRef]
- Nair, A.; Kuban, B.D.; Tuzcu, E.M.; Schoenhagen, P.; Nissen, S.E.; Vince, D.G. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 2002, 106, 2200–2206. [Google Scholar] [CrossRef]
- Ophir, J.; Cespedes, I.; Ponnekanti, H.; Yazdi, J.; Li, X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef] [PubMed]
- de Korte, C.L.; Pasterkamp, G.; van der Steen, A.F.; Woutman, H.A.; Bom, N. Characterization of plaque components with intravascular ultrasound elastography in human femoral and coronary arteries in vitro. Circulation 2000, 102, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Schaar, J.A.; deKorte, C.L.; Mastik, F.; Strijder, C.; Pasterkamp, G.; Boersma, E.; et al. Characterizing vulnerable plaque features with intravascular elastography. Circulation 2003, 108, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Doyley, M.M.; Mastik, F.; de Korte, C.L.; Carlier, S.C.; Cespedes, E.I.; Serruys, P.W.; et al. Advancing intravascular ultrasonic palpation toward clinical applications. Ultrasound Med Biol 2001, 27, 1471–1480. [Google Scholar] [CrossRef]
- Schaar, J.A.; Regar, E.; Mastk, F.; McFadden, E.P.; Saia, F.; Disco, C.; et al. Incidence of high strain patterns in human coronary arteries: assessment with three-dimensional intravascular palpography and correlation with clinical presentation. Circulation 2004, 109, 2716–2719. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Casscells, W.; Hathorn, B.; David, M.; Krabach, T.; Vaughn, W.K.; McAllister, H.A.; et al. Thermal detection of cellular infiltrates in living atherosclerotic plaques: possible implications for plaque rupture and thrombosis. Lancet 1996, 347, 1447–1451. [Google Scholar] [CrossRef]
- Stefanidis, C.; Diamantopulos, L.; Vlachopulos, C.; Tsiamis, E.; Dernellis, J.; Toutouzas, K.; et al. Thermal heterogeneity within human atherosclerotic coronary arteries detected in vivo: a new method of detection by application of a special thermography catheter. Circulation 1999, 99, 1965–1971. [Google Scholar] [CrossRef]
- Stefanadis, C.; Toutouzas, K.; Tsiamis, E.; Stratos, C.; Vavuranakis, M.; Kallikazaros, I.; et al. Increased local temperature in human coronary atherosclerotic plaques: an independent predictor of clinical outcome in patients undergoing a percutaneous coronary intervention. J Am Coll Cardiol 2001, 37, 1277–1283. [Google Scholar] [CrossRef]
- Yabushita, H.; Bouma, B.E.; Houser, S.L.; Aretz, T.; Jang, I.K.; Schlendorf, K.H.; et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002, 106, 1640–1645. [Google Scholar] [CrossRef]
- Jang, I.K.; Bouma, B.E.; Kang, D.H.; Park, S.J.; Park, S.W.; Seung, K.B.; et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 2002, 39, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Muller, J.E. Detection of high-risk atherosclerotic coronary plaques by intravascular spectroscopy. J Interv Cardiol 2003, 16, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Slager, C.J.; Wentzel, J.J.; Schuurbiers, J.C.H.; Oomen, J.A.F.; Kloet, J.; Krams, R.; et al. True 3-dimensional reconstruction of coronary arteries in patients by fusion of angiography and IVUS (ANGUS) and its quantitative validation. Circulation 2000, 102, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Wentzel, J.J.; Janssen, E.; Vos, J.; Schuurbiers, J.C.H.; Krams, R.; Serruys, P.W.; et al. Extension of increased wall thickness into high shear stress regions is associated with loss of compensatory remodeling. Circulation 2003, 108, 17–23. [Google Scholar] [CrossRef]
© 2004 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Agostoni, P.; Schaar, J.A.; Serruys, P.W. The Challenge of Vulnerable Plaque Detection in the Cardiac Catheterization Laboratory. Cardiovasc. Med. 2004, 7, 349. https://doi.org/10.4414/cvm.2004.01049
Agostoni P, Schaar JA, Serruys PW. The Challenge of Vulnerable Plaque Detection in the Cardiac Catheterization Laboratory. Cardiovascular Medicine. 2004; 7(10):349. https://doi.org/10.4414/cvm.2004.01049
Chicago/Turabian StyleAgostoni, Pierfrancesco, Johannes A. Schaar, and Patrick W. Serruys. 2004. "The Challenge of Vulnerable Plaque Detection in the Cardiac Catheterization Laboratory" Cardiovascular Medicine 7, no. 10: 349. https://doi.org/10.4414/cvm.2004.01049
APA StyleAgostoni, P., Schaar, J. A., & Serruys, P. W. (2004). The Challenge of Vulnerable Plaque Detection in the Cardiac Catheterization Laboratory. Cardiovascular Medicine, 7(10), 349. https://doi.org/10.4414/cvm.2004.01049



