1. Introduction
Since the introduction of transcatheter aortic valve implantation (TAVI) in Switzerland in 2007, starting with only 18 cases, an increasing trend has been observed, leading to a total of 2004 TAVI procedures in the year 2021 [
1]. This increase can be attributed to several factors, including the exponential growth in the prevalence of degenerative aortic valve stenosis and the aging demographic of the general population. Nowadays, advanced age plays a significant role in discussions within heart teams, as older patients are more likely to be considered for less invasive procedures. Consequently, TAVI has emerged as the preferred treatment approach for a wide range of elderly patients presenting with symptomatic severe aortic stenosis [
2].
In contrast to surgical aortic valve replacement, where the prosthetic valve is sewn into place, TAVI relies on the principle of unfolding and anchoring, which carries the inherent risk of periprocedural or delayed transcatheter valve embolization and migration (TVEM) [
3]. Predisposing risk factors for TVEM include anatomical factors such as bulky calcified leaflets, the presence of a bicuspid aortic valve, interference with left ventricular structures like severe ventricular hypertrophy or mitral prosthesis struts, and a horizontal aortic annulus (angulation > 48°). Technical factors contributing to the risk include sizing errors, high deployment, incomplete balloon inflation, pacing failure or premature pacing termination, postdilation of the transcatheter valve, failure to retract the delivery sheath, and resuscitation manoeuvres. Device-related factors include the use of self-expanding, first-generation, and resheathable devices, as well as manufacturing defects [
3,
4,
5].
A study using data from the TRAVEL (Transcatheter Heart Valve Embolization and Migration) registry, covering a total of 29,636 TAVI procedures performed between January 2010 and December 2017 across 26 participating centres in Europe and North America, identified 273 cases of TVEM (0.92%, ranging from 0.28 to 3.68% across all centres). Out of these cases, 217 (79.5%) involved embolization into the ascending aorta, while 56 (20.5%) resulted in embolization into the left ventricle, with the majority occurring either during or shortly after prosthesis placement [
3]. According to the limited data available, TVEM, although occurring rarely, is associated with a poor prognosis [
3]. The most frequently used definitive therapy has been the implantation of a second prosthesis, which has demonstrated a high degree of technical success and acceptable procedural outcomes [
3].
The standard pharmaceutical therapy after successful TAVI includes single antiplatelet therapy (SAPT) in patients with no baseline indications for oral anticoagulation (OAC) [
6]. Although OAC is not currently the standard of care after TAVI, it may need to be considered if certain thrombotic complications, such as valve thrombosis, cerebrovascular events, and atrial fibrillation, occur [
7]. To date, there are no established guidelines for the management of valve prosthesis embolization.
We report about the implantation of a second aortic valve prosthesis after periprocedural embolization of the first transcatheter valve, which resulted in postprocedural residual floating in the ascending aorta and postprocedural treatment with OAC in addition to monotherapy with antiplatelet agents.
2. Case Description
An 84-year-old female patient with severe aortic valve stenosis (maximal/mean rise of left ventricular pressure [dP max/mean] 81/53 mm Hg) without insufficiency, a TAVIcomputed tomography (CT) calcium score of 4024, and New York Heart Association (NYHA) functional classification Class III was admitted for an elective TAVI procedure.
The patient’s medical history included a recent diagnosis of coronary artery disease involving two vessels (50% stenosis of the right coronary artery [RCA] proximally with post-stenotic aneurysmal dilation of the mid RCA, 50% stenosis in the mid and apical left anterior descending artery, and in the diagonal branch), suspicion of an intraductal papillarymucinous neoplasm or fatty interdigitations as an incidental finding during a TAVI-CT, and suspicion of bronchial asthma. An electrocardiogram showed a pre-existing first-degree atrioventricular block.
With a EuroSCORE II (European system for cardiac operative risk evaluation) of 2.65%, the patient was classified as having a low perioperative risk, but given the patient’s advanced age, TAVI was considered to be the most appropriate approach.
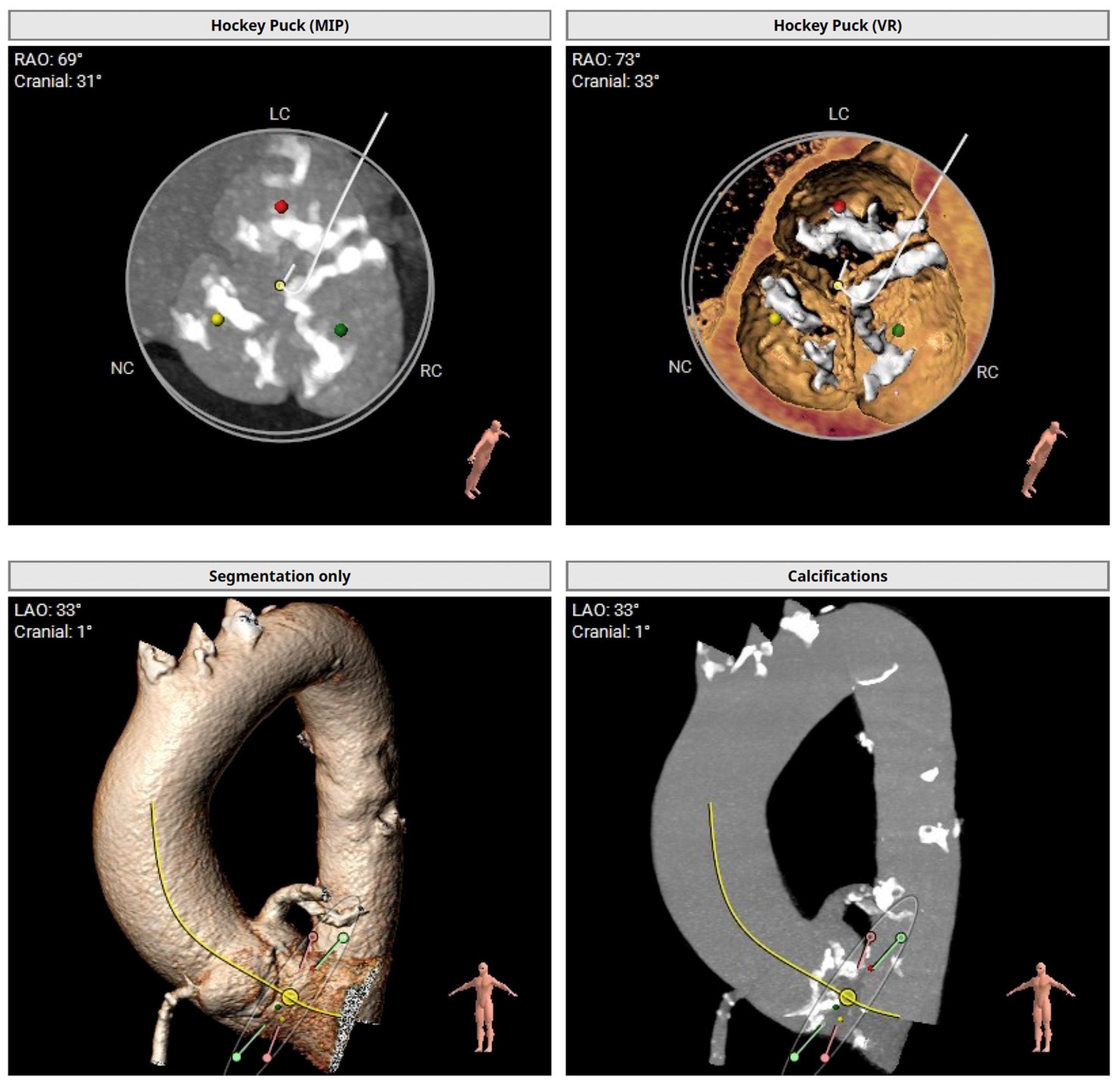
The preprocedural TAVI-CT showed an asymmetric calcium distribution with no calcification in the left ventricular outflow tract and a horizontal aortic annulus (
Figure 1). With an average aortic annulus diameter of 24 mm and a maximum of 29 mm, the Evolut™ PRO+ 29 mm valve (Medtronic Cardiovascular, Minneapolis, MN, USA) was selected for the procedure.
No significant conduction abnormalities were observed during the periprocedural period, but there were fluctuations in heart rate with bradycardia down to 30 beats per minute, necessitating the placement of a temporary pacemaker. During the first implantation attempt of an Evolut™ PRO+ 29 mm valve following standard technique through the right femoral artery, the prosthesis embolized in the ascending aorta. Due to the strongly horizontal aorta, severe calcification of the aortic arch, and the high risk of injuring the aorta during repositioning, the prosthesis had to be left in place. Due to anatomical circumstances, the prosthesis was not expected to compromise the extracranial feeding vessels or coronary arteries. Subsequently, the implantation of an Edwards SAPIEN 3 Ultra™ 26 mm valve (Edwards Lifesciences, Irvine, CA, USA) through the right femoral artery was performed without complications.
After the valve implantation was completed, an ultrasound examination was conducted to confirm the position of the valve. The initial echocardiographic follow-up showed a correctly positioned aortic valve prosthesis with normal function (mean gradient of 8 mm Hg) and no significant transvalvular regurgitation. The embolized valve prosthesis floated in the ascending aorta with a pulse-synchronous cranio-caudal translation of approximately 1.5 cm.
After recovery from the TAVI in the presence of clinical signs of heart failure, a chest X-ray and a repeat transthoracic echocardiography were performed to assess the position of the grafts (
Figure 2).
In the case of mild cardiac decompensation, short-term diuretic therapy with torasemide was administered. A follow-up echocardiogram demonstrated good systolic function (visually estimated left ventricular ejection fraction [LVEF] of 55%). The intact valve prosthesis remained in the correct position with normal function (mean gradient of 8 mm Hg, maximum aortic velocity [Vmax] of 2 m/s) and no trans- or paravalvular regurgitation. The embolized valve prosthesis continued to float within the ascending aorta. In direct comparison to the previous examination, the mobility of the embolized prosthesis was reduced, particularly in terms of craniocaudal movement, with only minimal lateral pulse-synchronous movement remaining. Postinterventional, a therapy regimen consisting of Apixaban and Aspirin® for a cumulative duration of six months was initiated. The rationale for this combined therapeutic approach with OAC and SAPT was based on the hypothesis that the embolized valve, with its apposition and residual pulsatile motion, poses an additional increased risk of thrombogenesis. Treatment with a non-vitamin K oral anticoagulant was chosen over vitamin K antagonists due to its lower susceptibility to interactions with food and other medications, as well as the elimination of the need for monitoring of the international normalized ratio (INR). Furthermore, treatment with angiotensin-converting enzyme (ACE) inhibitors and statins was initiated.
During the 2-month cardiological follow-up, the patient presented without dyspnea or symptoms of angina. Echocardiography showed preserved global systolic function (visually estimated LVEF of 55–60%). The gradients across the correctly implanted aortic valve were normal (dP max/mean 14/9 mm Hg), with no insufficiency. The incorrectly placed prosthesis in the ascending aorta continued to show a slight pulse-synchronous movement. The therapy with OAC and SAPT was continued.
3. Discussion
In our case, the embolism of a self-expanding TAVI prosthesis was treated by implanting a second prosthesis, while the first prosthesis remained in a floating position in the ascending aorta.
Treatment of valve embolisms into the aorta may differ depending on the type of valve; however, it traditionally relies on repositioning the valve to an appropriate supra- annular position. If a balloon-expandable valve embolizes, it should be tried to retract the valve into the descending aorta by retrieving a valvuloplasty balloon that has been inflated distally to the valve. If a self-expanding valve embolizes, in the majority of cases the valve cannot be safely retrieved from beyond the aortic arch because of the risk of stroke, so it is preferable to secure it at the level of the ascending aorta, above the sinotubular junction, to avoid compressing the aortic root and the supra-aortic trunks.
In our case, the embolized valve had to be left in place due to a strongly horizontal aorta, severe calcification of the aortic arch, and the high risk of inducing an aortic injury during repositioning.
Thrombotic events are a significant concern during and after TAVI procedures and are associated with various procedural- and valve-related factors. Antithrombotic therapy is necessary after TAVI to prevent thrombotic complications, but it raises the risk of bleeding [
7]. In patients without an established indication for OAC after successful TAVI, a treatment strategy including OAC was associated with a higher risk of death or thromboembolic complications and a higher risk of bleeding than an antiplatelet-based strategy [
8]. Research shows that the incidence of bleeding and the risk of major or life-threatening bleeding complications were significantly lower with SAPT compared to dual antiplatelet therapy, with no difference in the beneficial effect on ischemic events [
9,
10].
Therefore, the ESC/EACTS (European Society of Cardiology and European Association for Cardio-Thoracic Surgery) guidelines recommend a lifelong OAC therapy for TAVI patients who have other indications for OAC (Class I, Level of Evidence B) and a lifelong SAPT in patients with no baseline indication for OAC (Class I, Level of Evidence A). Finally, routine use of OAC after TAVI is not recommended in patients with no baseline indication for OAC (Class III, Level of Evidence B) [
6].
However, although TVEM has been described previously, there are currently neither guidelines on optimal pharmaceutical therapy nor research on whether a different follow-up schedule should be advised. The management of an embolized valve, especially when it involves residual movement, demonstrates the challenges involved in decision-making following a complication and illustrates the balancing act between reducing thrombotic risk and avoiding excessive bleeding. In our case, OAC in combination with SAPT was chosen solely as a precautionary measure, as we believe that the pulse-synchronous movement and apposition of the embolized valve prosthesis pose an increased risk of thromboembolic events. Therefore, in cases with residual valve movement, our advice is to consider prescribing anticoagulation. However, individual risk-benefit considerations should be taken into account.
This case also highlights the importance of a thorough preprocedural assessment, which includes not only the patient’s age and an assessment of intraoperative risk but also the evaluation of anatomical factors to ensure optimal device selection and valve sizing. Moreover, it emphasizes the need for additional monitoring to detect any late complications and to see whether a different follow-up schedule is needed.
4. Conclusions
The management of an embolized valve, particularly in the presence of residual motion, presents significant clinical decision-making challenges. The lack of established guidelines on optimal pharmaceutical therapy or follow-up schedules further complicates this process. In the case described the combination of OAC and SAPT was used as a precautionary measure due to the perceived increased risk of thromboembolic events from the embolized prosthetic valve with residual movement. This highlights the importance of individualised risk-benefit analysis in the management of such cases, particularly when considering anticoagulation therapy. In addition, the case highlights the critical need for thorough pre-procedural assessment, including consideration of patient-specific anatomical factors, to optimise device selection and sizing. Finally, it emphasises the need for ongoing monitoring to detect late complications and assess the potential need for revised follow-up protocols.