Introduction
The diagnosis of aortic stenosis (AS) marks the beginning of a lifelong journey. Management options for aortic stenosis are often spectrometric rather than dichotomous. While the evidence continues to evolve, from cellular pathology to population-based studies, unfolding new theories and discoveries, several important preventative and therapeutic challenges remain. In this article, we discuss some of the common challenges in the lifetime management of AS, the literature underpinning the current opinion and the existing gaps in the evidence.
Epidemiology
The exact prevalence and incidence of AS remain underdetermined due to a marked variability between the studies in terms of design, targeted populations, recruitment methods and diagnostic definitions of AS [
1,
2,
3]. Many studies predominantly pertain to symptomatic patients with a severe disease or the analysis of surgical databases, excluding asymptomatic patients, patients with a less than severe AS and those with significant comorbidities precluding surgical treatment [
4]. Further, genetic and socioeconomic factors also influence the prevalence of AS [
5].
In a pooled population-based study, 11,911 adults were assessed prospectively using echocardiography. Within this population, the prevalence of AS was 0.2% in individuals aged 55 to 64 years and rose sharply to 2.8% in individuals over 75 years old [
6]. In a large-scale community echocardiographic screening for undiagnosed valve disease in individuals aged ≥65 years in the United Kingdom, mild and significant (moderate/severe) calcific aortic valve disease was found in 34.6 and 0.7% of the screened population, respectively [
2]. Furthermore, several studies and registries have shown a consistent and significant association between age and the prevalence of AS [
1,
3,
4], forecasting an upward trend in the incidence of AS in the aging populations.
Pathophysiology and Natural History
A wealth of knowledge already exists about the pathophysiology of rheumatic and infectious aortic valve disease, but our knowledge about degenerative non-rheumatic aortic valve disease has evolved only recently. Degenerative non-rheumatic aortic valve disease is no longer attributed to mere passive wear and tear, but to a rather active inflammatory, infiltrative and proliferative process in which genetic and acquired factors are translated into the fibrocalcific remodeling of the valve [
7,
8]. The active pathological processes involved and the identified risk factors for calcific valve disease are analogous to those seen in atherosclerosis, sparking a contemporary belief that calcific aortic valve disease is modifiable through preventative methods and therapeutic targets [
7,
9].
The natural history of the disease is characterized by an asymptomatic latent period followed by a symptomatic phase with a predictable adverse outcome unless the valve is replaced [
10]. The progression rate of leaflet calcification into hemodynamically relevant AS and symptomatic disease varies significantly between individuals [
11].
Once hemodynamically significant, the natural history of AS becomes slightly more predictable, although considerable individuality remains. The hemodynamic progression of AS can be associated with extra-valvular cardiac damage, which adds significantly to the adversity of the disease and is strongly associated with clinical outcomes in both symptomatic and asymptomatic AS, even after the valve replacement [
12,
13]. This conceptualizes the contemporary shift from simple grading to a more outcome-associated staging of the AS and opens the debate on the optimal timing of intervention [
12].
Prevention of Disease Progression
In the quest for pharmacological agents to slow the progression of AS, efforts have been made to elucidate the molecular pathways linked to the pathogenesis of the disease. Among the key pathways involved, lipid metabolism, adiposity and inflammation have received significant attention as potential therapeutic targets [
8].
The statin’s pleiotropy extends to all pathways mentioned above, therefore, its potential effect on the progression of AS was probed in several studies. A meta-analysis of four randomized controlled trials (RCTs) examining the impact of statin therapy on the progression and clinical outcomes of mild to moderate AS showed no impact of statin therapy on the echocardiographic parameters or the clinical outcomes of AS (median follow-up 2.1–4.4 years), despite a significant reduction in LDL levels (36–55%) [
14]. Nevertheless, one of the trials included in the meta-analysis provided further insight into the role of oxidized phospholipids and their main carrier, lipoprotein(a), in the progression of AS. A follow-up study has shown that elevated lipoprotein(a) and oxidized phospholipids were strong predictors of the progression rate of AS [
15]. Furthermore, rosuvastatin therapy was associated with an annual increase of lipoprotein(a) and oxidized phospholipids by 20 and 46%, respectively [
15]. This further supports the lipoprotein(a) and oxidized phospholipids theory and may explain the failure of statin therapy to modify the course of AS.
Dedicated testing of the lipoprotein(a) and oxidized phospholipids hypothesis has been conducted in several studies, all of which have suggested a strong pro-inflammatory effect and an abundant presence in the diseased aortic valve [
16,
17,
18].
PCSK9 inhibition, a non-selective lipoprotein(a) lowering therapy, has shown a promising signal in slowing the progression of AS [
19], advocating a potential role for specific lipoprotein(a) and oxidized phospholipids lowering or targeting therapies in slowing the progression of AS, a concept that is currently being investigated in ongoing studies [
17].
Selective lipoprotein(a) lowering agents have been developed and investigated in patients with atherosclerotic disease. Olpasiran, a small interfering RNA that reduces lipoprotein(a) synthesis in the liver, is an effective circulating lipoprotein(a) lowering agent [
20], but its effect on cardiovascular disease is yet to be determined. Pelacarsen, an investigational specific lipoprotein(a) lowering agent that inhibits the production of apolipoprotein(a) in the liver, is currently being investigated in AS (NCT05646381). Meanwhile, there is no clinically approved medical treatment for halting the progression of AS. Similarly, the benefit of therapies that could modify the extra-valvular modeling or damage in the context of significant AS, especially before valve replacement, is not established.
Timing of Intervention
While it is universally recognized that symptomatic severe aortic valve stenosis is a clear and robust indication for valve replacement, intervening in asymptomatic severe disease and less than severe AS has been a debating point recently. As a general concept, we are transitioning from grading to staging of disease. While grading of AS largely relies on measurements of the aortic valve area and transvalvular gradients, staging of disease considers secondary cardiac damage resulting from the increase in afterload and includes the evaluation of left ventricular mass and function, left atrial dimensions, mitral and right ventricular damage, as well as the extent of pulmonary hypertension.
Severe Asymptomatic Aortic Stenosis
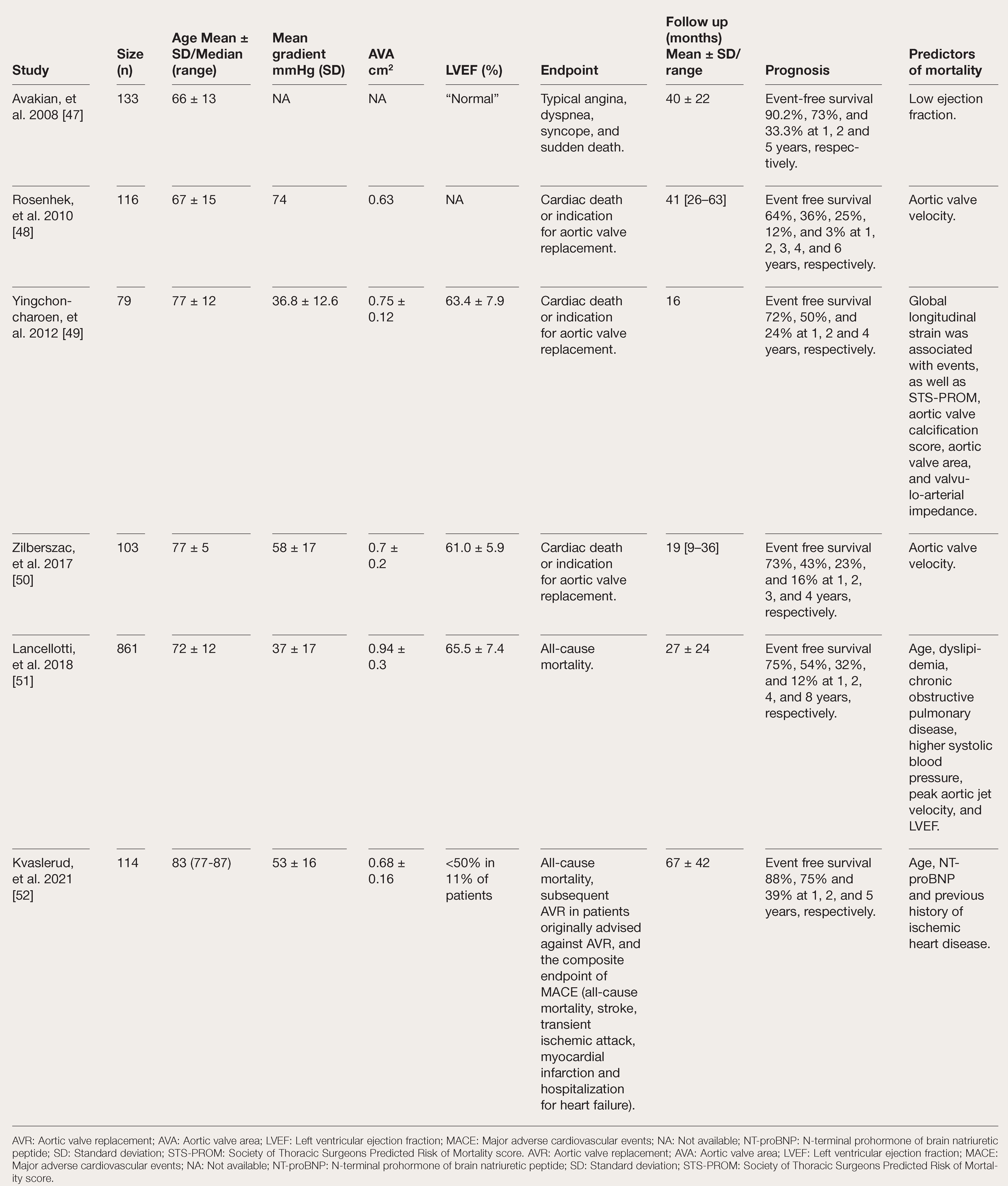
Asymptomatic AS is no longer considered a benign disease. Despite a relatively low risk of sudden cardiac death (0.2–3.1% per year) [
21], observational studies have shown rates of event-free survival (defined by freedom of allcause death without an indication for aortic valve replacement [AVR]) of around 70 and 25% at one and four years of follow-up, respectively, in patients with asymptomatic severe AS with preserved left ventricular systolic function (
Table 1).
RCTs have tried to seek a more objective verdict on the matter. The RECOVERY trial randomly assigned 145 asymptomatic patients with very severe AS (aortic valve area <0.75 cm2 plus aortic jet velocity >4.5 m/s or mean transcatheter gradient >50 mm Hg) to early surgery or conservative care. During an entire follow-up period of six years, the primary composite endpoint of operative mortality and cardiovascular mortality occurred in 0.7 and 15% in the early surgery and conservative care groups, respectively, driven solely by cardiovascular mortality [
22]. Patients in the RECOVERY trial were younger than 80 years of age (mean age 64 years), had a low prevalence of ischemic heart disease and a low predicted cardiac operative risk (EuroSCORE II 0.9%). A majority of patients had bicuspid aortic valve disease and the mean transvalvular gradient across the aortic valve was >60 mm Hg. Arguably, the benefit of early surgery may be less profound in patients with less severe AS.
Compared to RECOVERY, the AVATAR trial recruited 157 strictly asymptomatic patients (negative exercise tolerance test) with less critical and predominantly degenerative tricuspid AS with similarly low surgical risk. The rate of the primary composite endpoint of all-cause mortality, myocardial infarction, stroke and unplanned hospitalization for heart failure at three years was higher in the conservative treatment group compared to the early surgical group (34.7% vs 15.22%, hazard ratio 0.46, 95% confidence interval 0.23–0.9). However, the rate of cardiovascular death was comparable between the groups and rather interestingly, no non-cardiac death occurred in the early surgical group during the follow-up period compared to an 11% rate of non-cardiac deaths in the conservative management group [
23].
Trials recruiting older participants with more comorbidities and including transcatheter intervention would help shed more light on the management of asymptomatic severe AS. Several RCTs are underway (EVoLVeD NCT03094143, EARLY TAVR NCT03042104, ESTIMATE NCT02627391); meanwhile, observational studies reporting on the natural history of asymptomatic or less than severe AS remain invaluable in revealing prognostically important characteristics in patients with unoperated asymptomatic severe AS along with the outcomes.
Moderate Aortic Stenosis
The debate is also extended to less-than-severe AS. We are going through an evidence-backed transition from grading based solely on the severity of AS to staging, where the stage of the disease is stratified according to the presence and extent of cardiac damage, which is strongly associated with clinical outcomes [
12]. Recent observational literature signaled a superior survival with transcatheter AVR compared to medical therapy in patients with less than severe AS with reduced ejection fraction [
24]. This encouraged the instigation of RTCs that take a look at the benefits of AS intervention in moderate AS with symptoms (Evolute Expand TAVR II Pivotal Trial NCT 05149755), with evidence of cardiac damage (PROGRESS, NCT04889872), with heart failure (TAVR UN- LOAD NCT02661451) and with mitral regur- gitation (TIAMAR trial NCT05310461).
A comprehensive assessment of AS re- quires an integrative approach. Consideration of symptom status, parameters of AS severity, markers of myocardial injury and extra-valvu- lar imaging characteristics has the potential to optimize intervention timing and refine the risk stratification of AVR.
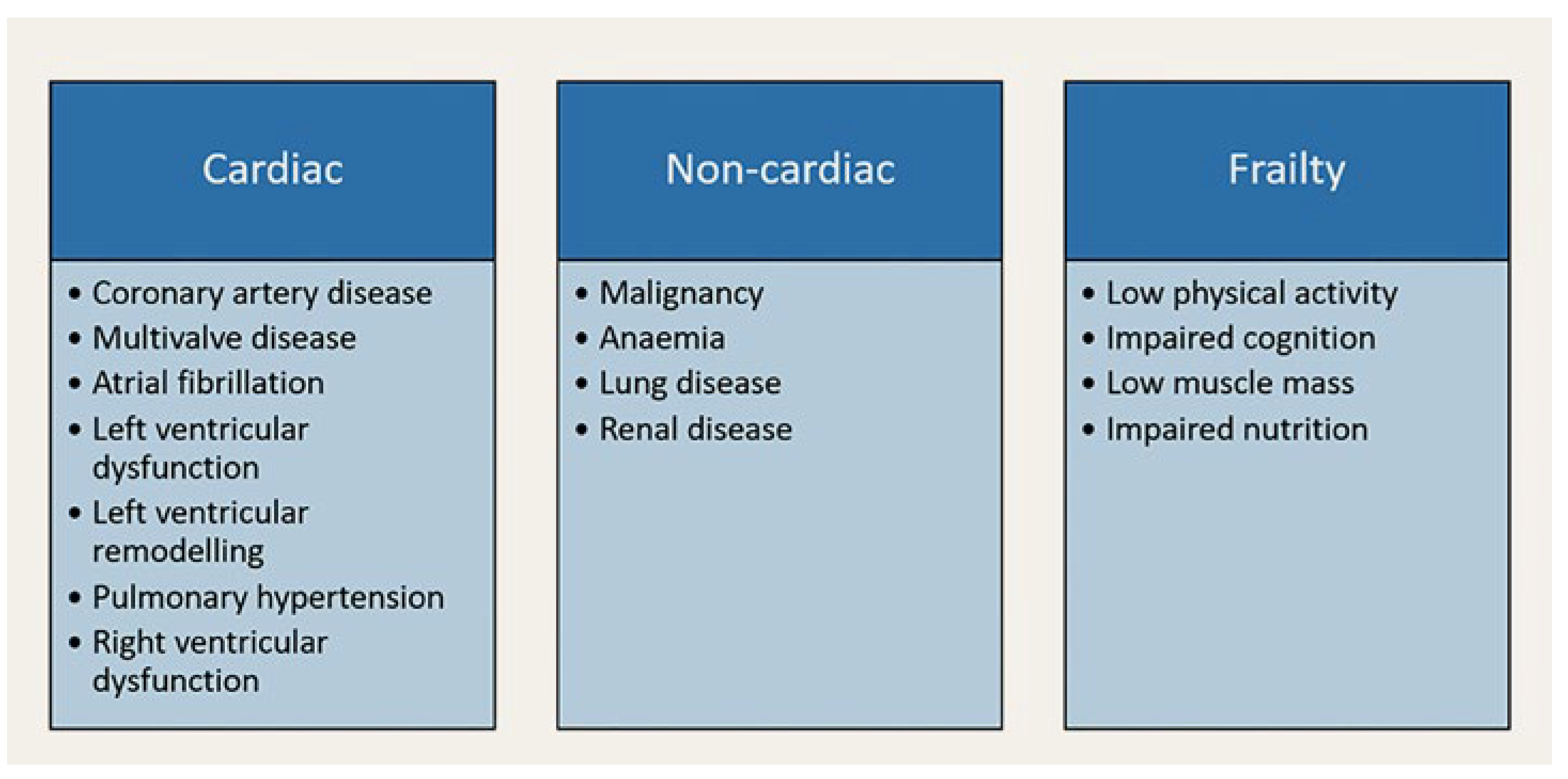
Quality of Life
A wealth of evidence suggests that aortic valve intervention offers both survival and quality of life benefits for the majority of pa- tients with symptomatic AS. However, outside the strict inclusion and exclusion criteria of landmark trials, the outcomes might be less comprehensive. In fact, up to one quarter of patients experience poor or no symptomatic or functional improvement one year after transcatheter aortic valve implantation (TAVI), even after a technically successful procedure [
25,
26]; a sobering reality that requires careful consideration. The priorities of individual patients with symptomatic severe AS are likely to be variable, with some prioritizing quality of life over quantity and others expecting the latter or aspiring to have both. Several studies looked at cardiac, extra-cardiac and frailty-related factors and conditions affecting the outcome of TAVI [
25,
27]. Additionally, several risk scores have been developed and validated for the prediction of short- and long-term mortality after TAVI, albeit with modest predictive power [
27]. However, only a limited number of reports focus specifically on the predictability of functional improvement after TAVI [
28].
Figure 1 lists some comorbidities that are suspected to negatively impact the functional outcomes of AV interventions.
Table 1.
Survival and Predictors of Outcomes in Asymptomatic Severe AS in Observational Studies.
Table 1.
Survival and Predictors of Outcomes in Asymptomatic Severe AS in Observational Studies.
It is likely that the lack of compensatory mechanisms and the degree and reversibility of cardiac damage affect the development of cardiac dysfunction and its regression after aortic valve intervention. However, while advanced cardiac damage is well validated as a strong predictor of mortality at one year [
13], in a cohort of 144 consecutive post-TA-VI patients, several parameters of cardiac damage at baseline did not appear to be associated independently with symptomatic nonresponse at one year post-TAVI [
28]. Intriguingly, low (<800 ng/l) or very high (>10 000 ng/l) baseline N-terminal prohormone of brain natriuretic peptide (NT-proBNP) values were strong independent predictors of symptom persistence at one year [
28], suggesting that a non-cardiac cause of disease or an advanced degree of cardiac damage might determine the degree of functional improvement after aortic valve intervention.
Further research is needed to identify other markers that could predict the futility of symptomatic relief. The value of those markers should be validated within multi-parameter risk scores. A holistic assessment of the patient’s symptoms and the quantification of the symptomatic contribution of each of the associated comorbidities helps to predict the reversibility of symptoms after aortic valve intervention. Above all, the patient’s priorities and expectations should be addressed and incorporated during the decision-making process.
Figure 1.
Comorbidities impacting the functional outcomes of aortic valve replacement.
Figure 1.
Comorbidities impacting the functional outcomes of aortic valve replacement.
Treatment Modalities
The treatment modality is determined by anatomic and non-anatomic feasibility, durability of the valve in relation to life expectancy and, if applicable, forward planning of iterative valve interventions. Middle-aged patients are primarily candidates for a mechanical AVR to reduce the risk of repeated interventions. The durability of mechanical aortic valves, with a reoperation rate as low as 3% within 15 years, comes at the expense of a lifelong commitment to anticoagulation therapy and the associated risks. However, lower rates of reoperation after mechanical compared to bioprosthetic AVR did not translate into a survival benefit with mechanical AVR in RCTs [
29,
30].
The Ross procedure is an alternative treatment modality to the mechanical AVR, particularly for young patients. In the Ross procedure, the aortic valve is replaced with the patient’s own pulmonary valve, while the latter is replaced by a pulmonary homograft. A meta-analysis including 13,129 children and young adults showed a low late mortality rate (0.54% and 0.59% per patient-year for children and adults, respectively) and a low rate of aortic autograft reintervention (1.28 and 0.83% per patient-year) [
32]. In the 2,444 patients (mean age 44.1 ± 11.7 years) included in the Ross registry up until 2018, 75.8% of the patients survived 25 years post procedure, with a low rate of aortic autograft intervention (0.69% per patient-year) and right ventricular outflow tract reintervention (0.62% per patient-year) [
32].
Yet another alternative to conventional surgical valve replacement is the aortic valve neocuspidization (AVNeo). During AVNeo, the three aortic cusps are independently replaced with natural-size glutaraldehyde-fixed autologous pericardial cusps [
33]. In 850 patients (median age 71 years, range: 13–90 years) who underwent AVNeo procedure (including 534 patients with AS), freedom from death, reoperation and recurrent moderate or greater aortic regurgitation was 85.9, 4.2 and 7.3%, respectively, within a mean follow-up period of 53.7 ± 28.2 months [
34]. Data on the long-term durability and outcome of this technically challenging procedure is not yet available. However, the low tissue reactivity could potentially be associated with a slower rate of degeneration and a more straightforward redo surgery [
35].
Bioprosthetic valve replacement, performed by a surgical or transcatheter approach, is the treatment of choice for elderly patients. A series of RCTs compared TAVI with surgical aortic valve replacement (SAVR) across the entire spectrum of surgical risk and established TAVI as a mainstay of treatment and a valuable alternative to SAVR in patients with suitable anatomy [
36]. A detailed discussion of TAVI versus SAVR in the treatment of AS goes beyond the scope of the present article. However, it is important to note that while the short-term outcomes of TAVI and SAVR are comparable, more light should be shed on the long-term implications of both treatment modalities in patients requiring valve-in-valve interventions.
The First Valve Sets the Stage for the Repeat Interventions
In a standard scenario, in addition to the surgical risk, several considerations are made in the decision-making process when choosing the first valve. Anatomical feasibility and challenges based on the implantation zone and implantation access are directly linked to the outcomes of the first implantation. Annulus dimensions, aortic leaflet, left ventricular outflow tract calcification, take-off of the coronary ostia, morphology of the sinus of Valsalva, aortic angulation, bicuspidity of the valve, vascular and alternative access, risk and relevance of coronary access restriction, and risk of conduction disturbance are all important considerations for the feasibility and challenges of TAVI, but less so for SAVR or surgically reconstructed autograft procedures. Other non-anatomical characteristics, such as suitability for anticoagulation considered contextually with preexisting comorbidities, compliance, lifestyle, occupation, family planning and patient preferences, are important when considering mechanical SAVR [
37].
After passing the feasibility test, the durability of the valve and the possibility of repeat interventions come into play. For candidates for a bioprosthesis who are expected to outlive their first valve, strategies for potential repeat interventions need to be considered upfront, at the time of the first valve implantation. The feasibility, risk and outcomes of second and subsequent repeat valve interventions are dictated by the modality of the first valve and the contemporary risk profile.
Figure 2 summarizes the potential issues associated with different strategies of sequential valvular interventions depending on the choice of the first and subsequent bioprosthetic valve. Outcomes and challenges of transcatheter valve-in-valve (ViV) implantation as a second or third valve strategy vary, depending on the modality and type of the preceding (initial) as well as the successive (subsequent) valve. Younger patients are likely to outlive the second bioprosthesis and to require a second or even third redo intervention, calling for even more thoughtful and cautious planning and consideration of the sequence of the implanted prostheses [
38].
Transcatheter Aortic Valve in Surgical Aortic Valve Implantation (TAV-in-SAV)
Clinical outcomes of TAV-in-SAV implantations depend on the mechanism of failure of the first valve and the valve size. Patients with AS as the mechanism of failure of the bioprosthetic valve have a higher mortality compared to patients with aortic regurgitation, and patients with small bioprosthetic valves have a higher mortality compared to those with large bioprosthetic valves [
39]. Two particular concerns in ViV implantation are the risk of coronary obstruction and the patient-prosthesis mismatch. Coronary obstruction can be differentiated mechanistically into direct and indirect obstruction. While the former is caused by an obstruction at the level of the coronary take-off, the latter is caused by the sequestration of the sinus at the level of the sinotubular junction. In addition to anatomical features of the aortic valvular complex such as valve-to-coronary distance and valve-to-sinotubular distance, the risk of coronary obstruction is determined by the design of the surgical bioprosthesis. Bioprostheses with externally mounted leaflets have the highest risk of coronary obstruction, followed by stentless bioprostheses and valves with internally mounted leaflets [
40]. Acute coronary obstruction occurs in 2 to 5% of TAV-in-SAV implantations and is caused by the neoskirt created by the permanently opened leaflets of the first valve prosthesis. In comparison, delayed coronary obstruction occurs in less than 1% of interventions and may be caused by continuing expansion of the transcatheter heart valve, thrombus formation, or fibrosis [
41].
Figure 2.
Clinical considerations with sequential aortic valve intervention. PPM: Patient-prothesis mismatch; SAVR: Surgical aortic valve replacement; SVD: Structural valve deterioration; TAVI: Transcatheter aortic valve implantation.
Figure 2.
Clinical considerations with sequential aortic valve intervention. PPM: Patient-prothesis mismatch; SAVR: Surgical aortic valve replacement; SVD: Structural valve deterioration; TAVI: Transcatheter aortic valve implantation.
Techniques for transcatheter electrosurgical leaflet modification, such as BASILICA (bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction), are effective in reducing the risk of coronary obstruction but are technically complex.
Patient-prosthesis mismatch is another concern in ViV implantation and is associated with a worse outcome. High residual gradients (mean gradient >20 mm Hg) were observed in 28% of patients in the Valve-in-Valve International Data (VIVID) registry and are of particular concern in patients with small bioprostheses [
39]. In these patients, ViV-TAVI with a supra-annular, self-expanding valve has been associated with better valve hemodynamics as compared to ViV-TAVI with an intra-annular, balloon-expandable valve [
42]. The bioprosthetic valve fracture technique using high-pressure balloon inflation has been developed to reduce patient-prosthesis mismatch but is associated with considerable periprocedural risk [
43].
Transcatheter Aortic Valve in Transcatheter Aortic Valve Implantation (TAV-in-TAV)
Compared to TAV-in-SAV, TAV-in-TAV has been associated with higher procedural success, numerically lower rates of coronary obstruction, lower residual gradients and a larger aortic valve area; however, 30-day and 1-year mortality rates were comparable between TAV-in-TAV and TAV-in-SAV [
44]. In contrast to surgical bioprostheses, the taller stent frame of transcatheter bioprostheses frequently extends beyond the take-off of the coronary arteries and may complicate coronary access with the implantation of a second transcatheter heart valve. Strategies for TAV-in-TAV depending on the initial and subsequent TAV type are a current matter of debate. Conceptually, supra-annular valves generate a higher neoskirt with the implantation of a second valve and thus a higher risk of coronary obstruction or inaccessibility. Therefore, a balloon-expandable valve is often preferred if the implantation of a second transcatheter valve is anticipated, particularly in patients with coronary artery disease. Alignment of the commissures of the first transcatheter heart valve with the native commissures facilitates coronary access after TAVI and allows for a BASILICA procedure prior to TAV-in-TAV implantation. Against this background, commissural alignment in the cusp overlap technique is the standard of care in contemporary TAVI if the cusp of the bioprosthesis is expected to extend beyond the take-off of the coronary arteries.
Redo Surgery and Explantation of Transcatheter Heart Valves
SAVR remains an alternative to ViV techniques. However, redo SAVR is associated with relatively high operative mortality (9.5%) and post-operative complications [
45]. Similarly, the risk of TAVI explantation cannot be dismissed. Neo-endothelialization of the stent frame of a transcatheter heart valve into the aortic wall requires intense endarterectomy and potentially extensive aortic root repair, for which special surgical techniques might be required. In the multicenter, international EXPLANT-TAVR registry, the in-hospital mortality rate post explantation was 11.9%, while the stroke rate was 5.9% [
46].
Conclusions
Evidence on the management of severe AS continues to evolve, but many gaps in our understanding of the disease, the optimal timing of intervention and the optimal sequence of treatment modalities remain. Upstream interventions for preventing or slowing down the progression of the disease have yet to be developed.
In the presence of several gaps in the evidence, the individuality of the cases should always be considered the norm rather than the exception, and management strategies should always be centered around patients’ expectations and priorities, guided by a holistic assessment of the disease and associated comorbidities, as well as the prognostic interplay between them.
Author Contributions
Bashir Alaour: Conception, design, writing of the first draft of the manuscript. Thomas Pilgrim: Conception, design and revision of the manuscript.
Conflicts of Interest
Thomas Pilgrim reports research grants to the institution from Biotronik, Boston Scientific, Edwards Lifesciences, and consultancy/speaker fees from Biotronik, Edwards Lifesciences, Abbott, Biosensors, Medtronic, and HighLife.
Recommended References
Bonow RO, Greenland P. Population-wide trends in aortic stenosis incidence and outcomes. Circulation. 2015 Mar;131(11):969–71.
d’Arcy JL, Coffey S, Loudon MA, Kennedy A, Pear-sonStuttard J, Birks J, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016 Dec;37(47):3515–22.
Martinsson A, Li X, Andersson C, Nilsson J, Smith JG, Sundquist K. Temporal trends in the incidence and prognosis of aortic stenosis: a nationwide study of the Swedish population. Circulation. 2015 Mar;131(11):988–94.
Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart. 2013 Mar;99(6):396–400.
Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al.; CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013 Feb;368(6):503–12.
References
- Bonow, R.O.; Greenland, P. Population-wide trends in aortic stenosis incidence and outcomes. Circulation. 2015, 131(11), 969–971. [Google Scholar] [CrossRef]
- d’Arcy, J.L.; Coffey, S.; Loudon, M.A.; Kennedy, A.; Pearson-Stuttard, J.; Birks, J.; et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016, 37(47), 3515–3522. [Google Scholar] [CrossRef]
- Martinsson, A.; Li, X.; Andersson, C.; Nilsson, J.; Smith, J.G.; Sundquist, K. Temporal trends in the incidence and prognosis of aortic stenosis: a nationwide study of the Swedish population. Circulation. 2015, 131(11), 988–994. [Google Scholar] [CrossRef]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart. 2013, 99(6), 396–400. [Google Scholar] [CrossRef]
- Thanassoulis, G.; Campbell, C.Y.; Owens, D.S.; Smith, J.G.; Smith, A.V.; Peloso, G.M.; et al. CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013, 368(6), 503–512. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: a population-based study. Lancet. 2006, 368(9540), 1005–1011. [Google Scholar] [CrossRef]
- Rajamannan, N.M. Calcific aortic valve disease: cellular origins of valve calcification. Arterioscler Thromb Vasc Biol. 2011, 31(12), 2777–2778. [Google Scholar] [CrossRef]
- Yu Chen, H.; Dina, C.; Small, A.M.; Shaffer, C.M.; Levinson, R.T.; Helgadóttir, A.; et al. Regeneron Genetics Center. Dyslipidemia, inflammation, calcification, and adiposity in aortic stenosis: a genome-wide study. Eur Heart J. 2023, 44(21) 1939. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Vassiliki’ Coutsoumbas, G.; Zagnoni, S.; Filippini, E.; Resciniti, E. Aortic stenosis in the elderly can be prevented: old risk factors and a new pathological condition. Eur Heart J Suppl. 2019, 21 Suppl B, B52–B53. [Google Scholar] [CrossRef]
- Kelly, T.A.; Rothbart, R.M.; Cooper, C.M.; Kaiser, D.L.; Smucker, M.L.; Gibson, R.S. Comparison of outcome of asymptomatic to symptomatic patients older than 20 years of age with valvular aortic stenosis. Am J Cardiol. 1988, 61(1), 123–130. [Google Scholar] [CrossRef]
- Généreux, P.; Stone, G.W.; O’Gara, P.T.; Marquis-Gravel, G.; Redfors, B.; Giustino, G.; et al. Natural History, Diagnostic Approaches, and Therapeutic Strategies for Patients With Asymptomatic Severe Aortic Stenosis. J Am Coll Cardiol. 2016, 67(19), 2263–2288. [Google Scholar] [CrossRef]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017, 38(45), 3351–3358. [Google Scholar] [CrossRef]
- Okuno, T.; Heg, D.; Lanz, J.; Praz, F.; Brugger, N.; Stortecky, S.; et al. Refined staging classification of cardiac damage associated with aortic stenosis and outcomes after transcatheter aortic valve implantation. Eur Heart J Qual Care Clin Outcomes. 2021, 7(6), 532–541. [Google Scholar] [CrossRef]
- Teo, K.K.; Corsi, D.J.; Tam, J.W.; Dumesnil, J.G.; Chan, K.L. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients. Can J Cardiol. 2011, 27(6), 800–808. [Google Scholar] [CrossRef]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bossé, Y.; Dumesnil, J.G.; et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J Am Coll Cardiol. 2015, 66(11), 1236–1246. [Google Scholar] [CrossRef]
- Yu, B.; Hafiane, A.; Thanassoulis, G.; Ott, L.; Filwood, N.; Cerruti, M.; et al. Lipoprotein(a) Induces Human Aortic Valve Interstitial Cell Calcification. JACC Basic Transl Sci. 2017, 2(4), 358–371. [Google Scholar] [CrossRef]
- Tsimikas, S. Potential Causality and Emerging Medical Therapies for Lipoprotein(a) and Its Associated Oxidized Phospholipids in Calcific Aortic Valve Stenosis. Circ Res. 2019, 124(3), 405–415. [Google Scholar] [CrossRef]
- Girard, A.; Gaillard, E.; Puri, R.; Capoulade, R.; Chan, K.L.; Paulin, A.; et al. Impact of C-reactive protein levels on lipoprotein(a)-associated aortic stenosis incidence and progression. Eur Heart J Open. 2023, 3(2), oead032. [Google Scholar] [CrossRef]
- Bergmark, B.A.; O’Donoghue, M.L.; Murphy, S.A.; Kuder, J.F.; Ezhov, M.V.; Ceška, R.; et al. An Exploratory Analysis of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibition and Aortic Stenosis in the FOURIER Trial. JAMA Cardiol. 2020, 5(6), 709–713. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; et al. OCEAN(a)-DOSE Trial Investigators. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N Engl J Med. 2022, 387(20), 1855–1864. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Hayward, C.; Pepper, J.; Senior, R. Risk stratification in asymptomatic severe aortic stenosis: a critical appraisal. Eur Heart J. 2012, 33(19), 2377–2387. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, S.W. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. Reply [Reply]. N Engl J Med. 2020, 383(1), 92–93. [Google Scholar]
- Afshar, M.; Yazdan-Ashoori, S.; Engert, J.C.; Thanassoulis, G. Drugs for Prevention and Treatment of Aortic Stenosis: How Close Are We? Can J Cardiol. 2021, 37(7), 1016–1026. [Google Scholar] [CrossRef]
- Ludwig, S.; Schofer, N.; Abdel-Wahab, M.; Urena, M.; Jean, G.; Renker, M.; et al. Transcatheter Aortic Valve Replacement in Patients With Reduced Ejection Fraction and Nonsevere Aortic Stenosis. Circ Cardiovasc Interv. 2023, 16(5), e012768. [Google Scholar] [CrossRef]
- Arnold, S.V.; Spertus, J.A.; Vemulapalli, S.; Li, Z.; Matsouaka, R.A.; Baron, S.J.; et al. Quality-of-Life Outcomes After Transcatheter Aortic Valve Replacement in an Unselected Population: A Report From the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017, 2(4), 409–416. [Google Scholar] [CrossRef]
- Puri, R.; Iung, B.; Cohen, D.J.; Rodés-Cabau, J. TAVI or No TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J. 2016, 37(28), 2217–2225. [Google Scholar] [CrossRef]
- Patel, K.P.; Treibel, T.A.; Scully, P.R.; Fertleman, M.; Searle, S.; Davis, D.; et al. Futility in Transcatheter Aortic Valve Implantation: A Search for Clarity. Interv Cardiol. 2022, 17, e01. [Google Scholar] [CrossRef]
- Allen, C.J.; Joseph, J.; Patterson, T.; Hammond-Haley, M.; McConkey, H.Z.; Prendergast, B.D.; et al. Baseline NT-proBNP Accurately Predicts Symptom Response to Transcatheter Aortic Valve Implantation. J Am Heart Assoc. 2020, 9(23), e017574. [Google Scholar] [CrossRef]
- Oxenham, H.; Bloomfield, P.; Wheatley, D.J.; Lee, R.J.; Cunningham, J.; Prescott RJet, a.l. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart. 2003, 89(7), 715–721. [Google Scholar] [CrossRef]
- Stassano, P.; Di Tommaso, L.; Monaco, M.; Iorio, F.; Pepino, P.; Spampinato Net, a.l. Aortic valve replacement: a prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J Am Coll Cardiol. 2009, 54(20), 1862–1868. [Google Scholar] [CrossRef]
- Etnel, J.R.; Grashuis, P.; Huygens, S.A.; Pekbay, B.; Papageorgiou, G.; Helbing, W.A.; et al. The Ross Procedure: A Systematic Review, Meta-Analysis, and Microsimulation. Circ Cardiovasc Qual Outcomes. 2018, 11(12), e004748. [Google Scholar] [CrossRef]
- Aboud, A.; Charitos, E.I.; Fujita, B.; Stierle, U.; Reil, J.C.; Voth, V.; et al. Long-Term Outcomes of Patients Undergoing the Ross Procedure. J Am Coll Cardiol. 2021, 77(11), 1412–1422. [Google Scholar] [CrossRef]
- Ozaki, S.; Kawase, I.; Yamashita, H.; Uchida, S.; Nozawa, Y.; Matsuyama, T.; et al. Aortic valve reconstruction using self-developed aortic valve plasty system in aortic valve disease. Interact Cardiovasc Thorac Surg. 2011, 12(4), 550–553. [Google Scholar] [CrossRef]
- Ozaki, S.; Kawase, I.; Yamashita, H.; Uchida, S.; Takatoh, M.; Kiyohara, N. Midterm outcomes after aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg. 2018, 155(6), 2379–2387. [Google Scholar] [CrossRef]
- Feindel, C.; Naimark, A. What goes around comes around…possibly. J Thorac Cardiovasc Surg. 2018, 155(6), 2377–2378. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022, 43(7), 561–632. [Google Scholar] [CrossRef]
- Head, S.J.; Çelik, M.; Kappetein, A.P. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017, 38(28), 2183–2191. [Google Scholar] [CrossRef]
- Windecker, S.; Okuno, T.; Unbehaun, A.; Mack, M.; Kapadia, S.; Falk, V. Which patients with aortic stenosis should be referred to surgery rather than transcatheter aortic valve implantation? Eur Heart J. 2022, 43(29), 2729–2750. [Google Scholar] [CrossRef]
- Dvir, D.; Webb, J.; Brecker, S.; Bleiziffer, S.; Hildick-Smith, D.; Colombo, A.; et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation. 2012, 126(19), 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.B.; Rodés-Cabau, J.; Blanke, P.; Leipsic, J.; Kwan Park, J.; Bapat, V.; et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J. 2018, 39(8), 687–695. [Google Scholar] [CrossRef]
- Jabbour, R.J.; Tanaka, A.; Finkelstein, A.; Mack, M.; Tamburino, C.; Van Mieghem, N.; et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018, 71(14), 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Abbas, A.E.; Serra, V.; Vilalta, V.; Nombela-Franco, L.; Regueiro, A.; et al. Balloon- vs Self-Expanding Valve Systems for Failed Small Surgical Aortic Valve Bioprostheses. J Am Coll Cardiol. Erratum in J Am Coll Cardiol. 2022, 80 (14), 1419. 2022, 80(7), 681–693. [Google Scholar] [CrossRef]
- Saxon, J.T.; Allen, K.B.; Cohen, D.J.; Chhatriwalla, A.K. Bioprosthetic Valve Fracture During Valve-in-valve TAVR: bench to Bedside. Interv Cardiol. 2018, 13(1), 20–26. [Google Scholar] [CrossRef]
- Landes, U.; Sathananthan, J.; Witberg, G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; et al. Transcatheter Replacement of Transcatheter Versus Surgically Implanted Aortic Valve Bioprostheses. J Am Coll Cardiol. 2021, 77(1), 1–14. [Google Scholar] [CrossRef]
- François, K.; De Backer, L.; Martens, T.; Philipsen, T.; Van Belleghem, Y.; Bové, T. Repeat aortic valve surgery: contemporary outcomes and risk stratification. Interact Cardiovasc Thorac Surg. 2021, 32(2), 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V.N.; Zaid, S.; Fukuhara, S.; Saha, S.; Vitanova, K.; Kiefer, P.; et al. EXPLANT-TAVR Investigators. Surgical Explantation After TAVR Failure: Mid-Term Outcomes From the EXPLANT-TAVR International Registry. JACC Cardiovasc Interv. 2021, 14(18), 1978–1991. [Google Scholar] [CrossRef] [PubMed]
- Avakian, S.D.; Grinberg, M.; Ramires, J.A.; Mansur, A.P. Outcome of adults with asymptomatic severe aortic stenosis. Int J Cardiol. 2008, 123(3), 322–327. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek, R.; Zilberszac, R.; Schemper, M.; Czerny, M.; Mundigler, G.; Graf, S.; et al. Natural history of very severe aortic stenosis. Circulation. 2010, 121(1), 151–156. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Gibby, C.; Rodriguez, L.L.; Grimm, R.A.; Marwick, T.H. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging. 2012, 5(6), 719–725. [Google Scholar] [CrossRef]
- Zilberszac, R.; Gabriel, H.; Schemper, M.; Laufer, G.; Maurer, G.; Rosenhek, R. Asymptomatic Severe Aortic Stenosis in the Elderly. JACC Cardiovasc Imaging. 2017, 10(1), 43–50. [Google Scholar] [CrossRef]
- Lancellotti, P.; Magne, J.; Dulgheru, R.; Clavel, M.A.; Donal, E.; Vannan, M.A.; et al. Outcomes of Patients With Asymptomatic Aortic Stenosis Followed Up in Heart Valve Clinics. JAMA Cardiol. 2018, 3(11), 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Kvaslerud, A.B.; Santic, K.; Hussain, A.I.; Auensen, A.; Fiane, A.; Skulstad, H.; et al. Outcomes in asymptomatic, severe aortic stenosis. PLoS One. 2021, 16(4), e0249610. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.