Introduction
Leadless pacemaker therapy has been developed in recent years to overcome potential transvenous leadand pocket-related complications. Compared to conventional transvenous devices, there is a more than 50% reduction in device complications together with a high implantation success rate according to registry analyses [
1,
2,
3]. However, leadless pacing also carries potential complications related to the implantation process. Pericardial tamponade and groin-related complications, such as local hematoma, arteriovenous fistula or arterial pseudoaneurysm, were encountered at a risk of around 1%. Furthermore, stenosis or tortuous anatomy of the inferior vena cava can prevent a successful femoral leadless pacemaker implantation [
1,
2]. Hence, other access sites may be necessary for the implantation. A first series of 82 patients from the Netherlands demonstrated that the implantation via the internal jugular venous route is a safe and efficient way for leadless pacemakers [
4]. The aim of this analysis is to investigate the first experience of jugular leadless pacemaker implantation in Switzerland.
Methods
This prospective analysis includes ten consecutively enrolled patients who underwent jugular leadless pacemaker implantation from 09/2022 to 12/2022 at the University Hospital Zurich. There was no contraindication for these patients to have a standard femoral implantation procedure, which was important because if the jugular access had failed, a femoral implantation was the predefined bailout strategy. Follow-up was conducted by assessing all available patient records from patient visits, hospitalizations, as well as device interrogation and tracings. The interventional and electrical parameters of the jugular approach were compared with the first 100 patients at our institution who underwent a femoral leadless pacemaker implantation from 2015 to 2019. Follow-up was three months for all patients. The study was approved by the local ethical committee (BASEC-Nr.: 2021-00930).
Statistics
Continuous variables were reported as mean +/standard deviation. Categorical variables were reported as counts (percentage). Distribution of normality was assessed with the Shapiro-Wilk test. Comparisons between categorical variables were performed through contingency tables and assessed using a chi-squared or Fisher’s exact test, as appropriate. Mann-Whitney U Test was used for comparison of the continuous variables which were not normally distributed. Statistical analysis was performed using IBM SPSS Statistics (Version 23).
Implantation Procedure
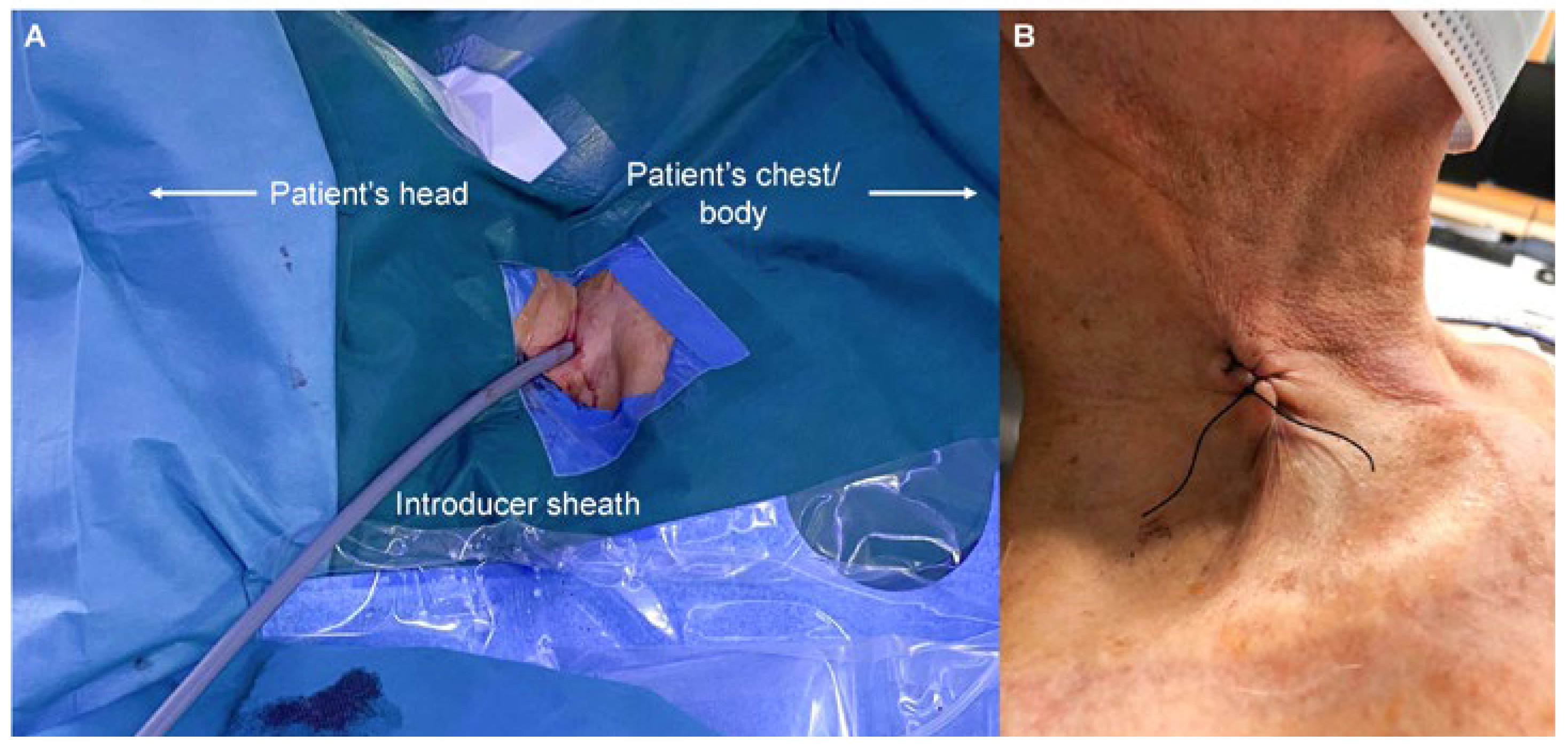
All implantations were performed in an electrophysiology laboratory. Sedation was not necessary except an initial bolus of 25–50 µg fentanyl. Perioperative infection prevention using cefuroxime was given to all patients. Skin disinfection was performed at the area between the two heads of the sternocleidomastoid muscle and the clavicle. After the patient was draped, 1% lidocaine was injected under the skin using a small subcutaneous needle. Puncture of the right internal jugular vein was guided by ultrasound with a linear probe in all patients. A standard J-wire was introduced and advanced under fluoroscopy guidance into the inferior vena cava. A one centimeter skin incision was carefully performed, as for the femoral access, and a standard 9 French sheath introduced over the J-wire. The J-wire was then replaced by an Amplatz Super-Stiff™ 0.035inch guidewire. The puncture site was predilated using a 20 French dilator followed by advancing the 27 French introducer sheath (Medtronic, Minneapolis, MN, USA) carefully to the level of the lower right atrium (video 1 and 2,
Figure 1A). The sheath was flushed, connected to a heparinized saline drip and a bolus of 3000–5000 units of heparin was injected. A sterile table was placed at the right superior site of the patient’s head to ease sheath and device handling. The delivery tool was advanced until the distal end of the sheath and the latter retracted back to the level of the superior vena cava (video 3). Right and left anterior oblique fluoroscopy views were used to cross the tricuspid valve and to maneuver the device within the right ventricle. As for the femoral approach, the goal was to place the device at the septal area of the right ventricle. Optimal septal location of device was confirmed in LAO view and by injecting contrast dye (video 4 and 5). If a satisfactory position was found, the device was deployed and electrical parameters were measured (adequate values were a pacing threshold <1 V at 0.24 ms and a sensed R-wave amplitude of >5 mV). Adequate fixation of the leadless pacemaker was verified with the pull and hold test (video 6). When at least two out of four tines were engaged with the myocardium, the tether was cut, and the device released. The delivery tool as well as the sheath were removed. The puncture site was closed with a modified Z-suture (
Figure 1B). Immediately after closing the puncture site, the interventional table was tilt into a reversed Trendelenburg position to reduce the venous pressure at the neck and to avoid the risk for bleeding.
Results
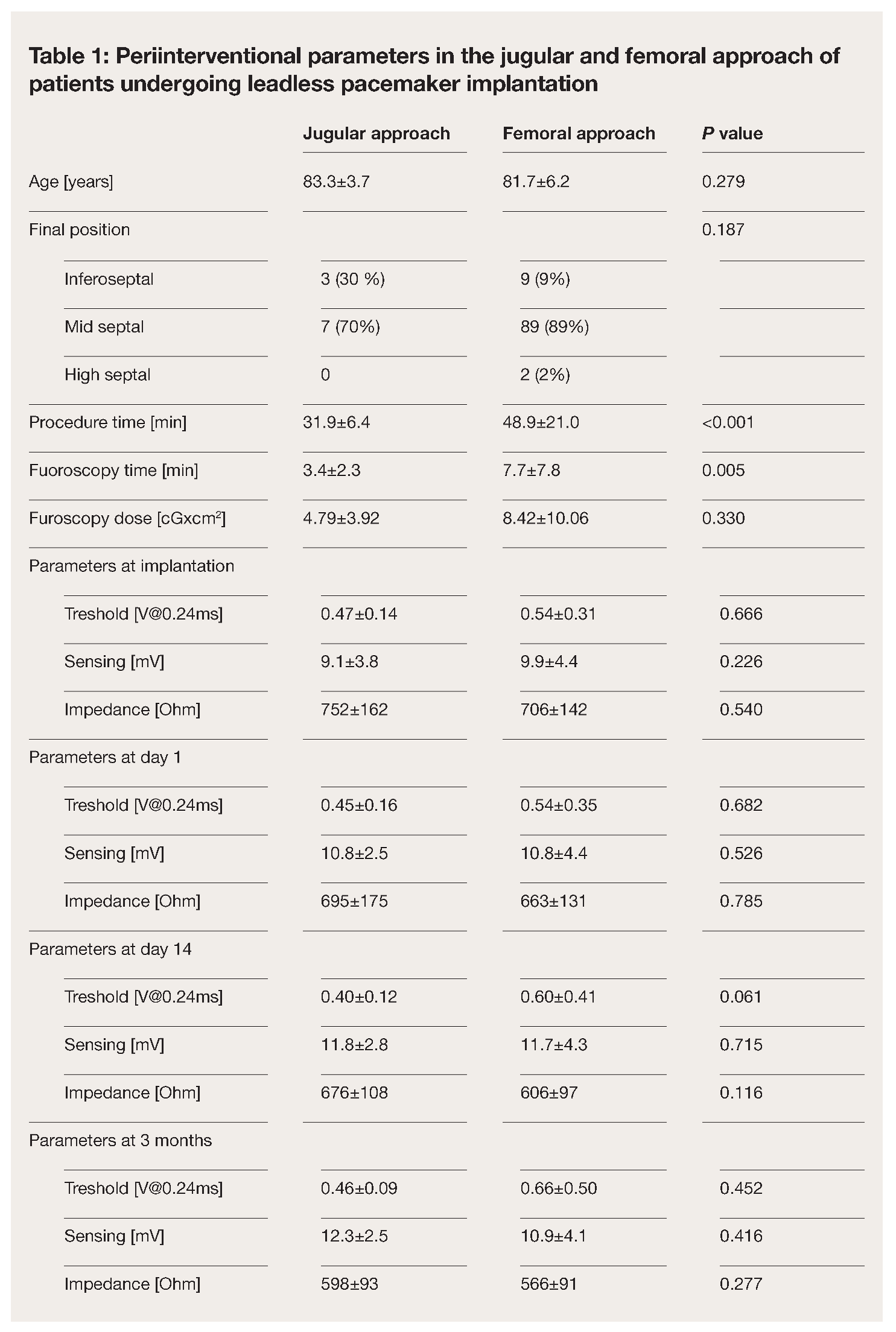
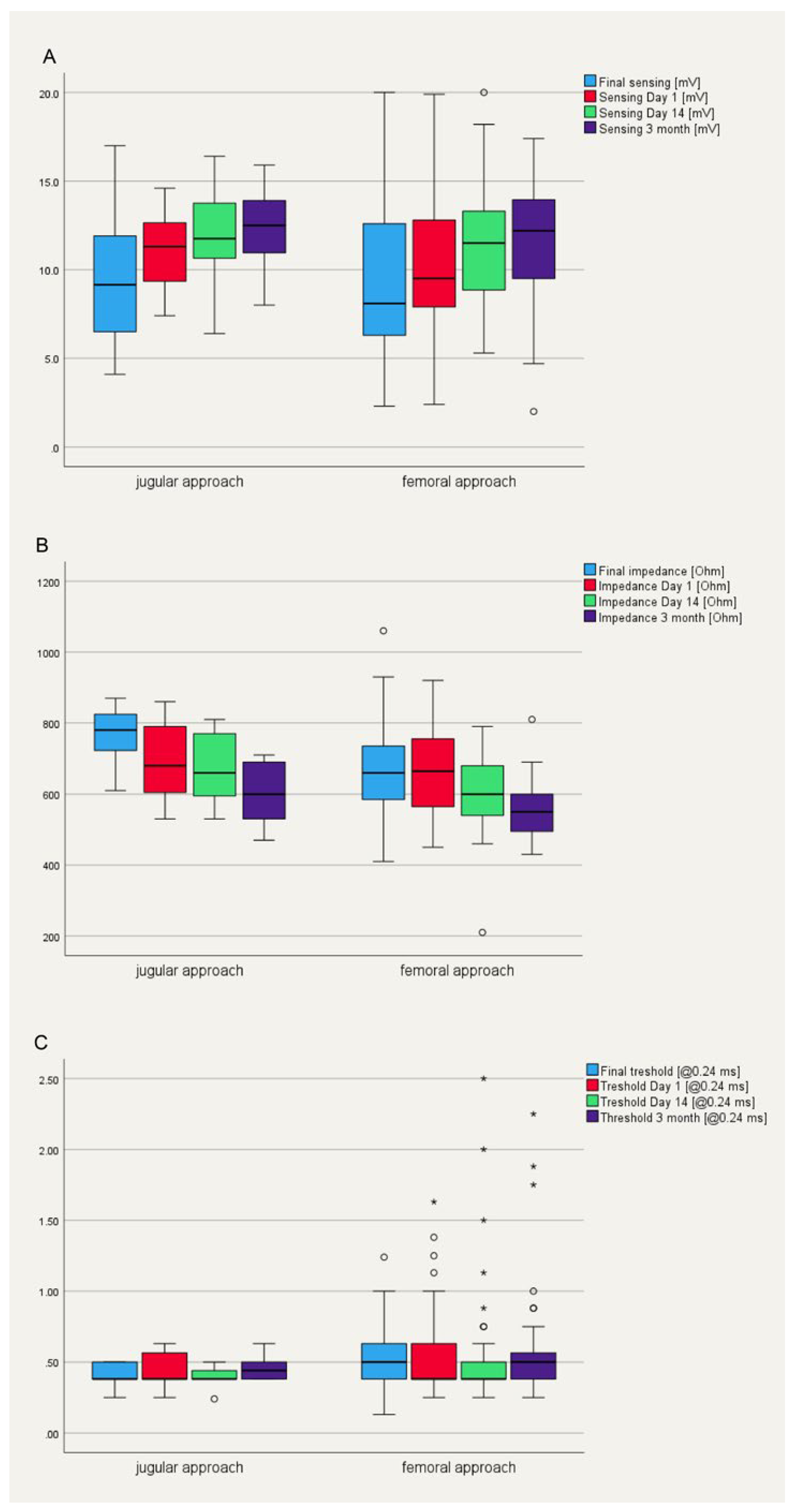
Patient’s characteristics are summarized in table 1 and compared to the first 100 patients who underwent a femoral leadless pacemaker implantation at our institution. Mean age of the ten patients was 83.3 ± 3.7 years. 60% (n=6) were male. Main indication for pacemaker implantation was AV block in 70% (n=7) of the patients, followed by tachy-brady syndrome in two patients with permanent atrial fibrillation and one patient with post conversion pauses. All devices were implanted successfully via the jugular approach. None of the patients experienced discomfort or a vagal reaction during access site dilatation or sheath advancement into the right atrium. Mean procedure time was 31.9 ± 6.4 min with a mean fluoroscopy time of 3.4 ± 2.3 min, which is significantly shorter compared to the femoral approach (procedure time 48.9 ± 21.0 min and fluoroscopy duration 7.7 ± 7.8 min, p < 0.01, Table 1). The device was positioned at the inferior septum in three patients and midseptal in seven patients. In seven patients, a sufficient device position was obtained at the first attempt, in two at the second, and in one at the fourth attempt (Table 1). The mean final pacing threshold in the end of the procedure was 0.47 ± 0.14 V at 0.24 ms pulse width with a ventricular sensing of 9.1 ± 3.8 mV and an impedance of 752 ± 162 Ohm (Table 1,
Figure 2). There were no periinterventional complications. At the first day after implantation, electrical parameters remained stable in all patients with a mean pacing threshold of 0.45 ± 0.16 V at 0.24 ms and a sensed amplitude of 10.8 ± 2.5 mV (Table 1,
Figure 2). At three months follow-up, all measured device parameters remained unchanged (Table 1,
Figure 2).
![Cardiovascmed 26 00194 i001 Cardiovascmed 26 00194 i001]()
Discussion
The aim of this case series was to evaluate the feasibility and safety of a jugular approach for leadless pacemaker implantation. In our experience from the first ten patients, this access site seems to be as safe and effective as the standard femoral approach.
The largest experience with jugular leadless pacemaker implantation originates from the Netherlands. Indeed, a recently published article summarizing their experience in 82 patients confirmed that the jugular approach for leadless pacemaker implantation seems to be a feasible and safe alternative to the femoral approach [
4]. Similarly to our case series, there were no puncture site related complications such as hematoma, arteriovenous fistula, or arterial aneurysm, which are some of the most encountered complications with the femoral approach [
4]. A possible explanation is that in most implantation procedures, ultrasound-guidance was used to puncture the internal jugular vein. Visualization of the puncture side is known to reduce potential complications [
5,
6] when inserting central venous lines. Furthermore, patients are encouraged to sit up immediately after the intervention, which is not only more convenient for the elderly population, but significantly reduces the venous pressure at the neck level and therefore the risk of local hematoma.
Another advantage is the straight and shorter route into the right atrium compared to the femoral vein. Occasionally, discomfort and vagal responses are observed when advancing the straight 27 French sheath through the more tortuous femoral and iliac veins due to mechanical stretch. Such a patient’s response has not been observed with the jugular approach increasing patient’s satisfaction during the intervention.
The procedure time is comparable to earlier studies of a standard leadless pacemaker implantation with a range from 26 to 41 minutes [
7,
8,
9,
10,
11]. The mean fluoroscopy time was similar to the Dutch experience [
4], but shorter than in studies using femoral access where fluoroscopy times equaled 6–13 min [
7,
9,
10], and also shorter in comparison to the first hundred femoral leadless pacemaker implantations at our institution. This difference can partly be explained by the shorter and straighter route into the patient’s heart as well as by the easier angulation towards the right ventricular septum, but it may be biased by the fact that the operators are already experienced in standard implantation of leadless pacemakers.
The device was placed in all patients at the right ventricular septum, avoiding an apical position. The more anterior position of the superior vena cava in comparison to the inferior vein, as well as the natural shape of the delivery catheter allow a better and easier angulation towards the ventricular septum. In most of the cases only one attempt was necessary to gain a stable and electrical favorable position at the ventricular septum. However, one need to keep in mind that the shorter and straighter route to the right ventricle from above can result in a higher translation of the forward pressure to the distal tip of the delivery tool when advancing the device. While from femoral approach usually only 10% of the forward pressure is translated directly to the end of the delivery catheter, the jugular approach eases this, resulting in a better contact with the myocardium, but potentially also carries a risk for more perforations. Even though this has not been observed in our cohort nor in the Dutch registry [
4], it is of paramount importance to adequately use different fluoroscopy angulations during the implantation procedure.
During a follow-up of three months, all pacing parameters remained stable or improved slightly, which is in line with previous published data of patients undergoing femoral leadless pacemaker implantation [
2].
Conclusion
From our experience of the ten first jugular leadless pacemaker implantations we can conclude that this approach of device implantation is a safe and effective alternative to the standard procedure with a high level of patient’s satisfaction, both during as well as directly after the intervention.
Ethics Statement
The study was approved by the local ethical committee (BASEC-Nr.: 2021-00930). Written informed consent has been obtained for the publication of
Figure 1.
Conflicts of Interest
AB has received consultant and/or speaker fees from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cook Medical, Daiichi Sankyo, Medtronic, Pfizer, and Spectranetics/Philipps. Cook Medical also supported him in attending meetings. NM declares no financial support and no other potential conflict of interest.
Data Availability Statement
Original data are available on request.
References
- Reynolds, D.; Duray, G.Z.; Omar, R.; Soejima, K.; Neuzil, P.; Zhang, S.; et al. Micra transcatheter Pacing Study Group. A Leadless Intracardiac Transcatheter Pacing System. N Engl J Med. 2016, 374, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Duray, G.Z.; Ritter, P.; El-Chami, M.; Narasimhan, C.; Omar, R.; Tolosana, J.M.; et al. Micra Transcatheter Pacing Study Group. Long-term performance of a transcatheter pacing system: 12-Month results from the Micra Transcatheter Pacing Study. Heart Rhythm. 2017, 14, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Stromberg, K.; Jackson, K.P.; Laager, V.; Duray, G.Z.; El-Chami, M.; et al. Micra Transcatheter Pacing Study Group. Long-term outcomes in leadless Micra transcatheter pacemakers with elevated thresholds at implantation: Results from the Micra Transcatheter Pacing System Global Clinical Trial. Heart Rhythm. 2017, 14, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Saleem-Talib, S.; van Driel, V.J.; Nikolic, T.; van Wessel, H.; Louman, H.; Borleffs, C.J.; et al. The jugular approach for leadless pacing: A novel and safe alternative. Pacing Clin Electrophysiol. 2022, 45, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Teichgräber, U.K.; Benter, T.; Gebel, M.; Manns, M.P. A sonographically guided technique for central venous access. AJR Am J Roentgenol. 1997, 169, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Randolph, A.G.; Cook, D.J.; Gonzales, C.A.; Pribble, C.G. Ultrasound guidance for placement of central venous catheters: A meta-analysis of the literature. Crit Care Med. 1996, 24, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Della Tommasina, V.; Barletta, V.; Di Cori, A.; Rogani, S.; Viani, S.; et al. Feasibility and long-term effectiveness of a non-apical Micra pacemaker implantation in a referral centre for lead extraction. Europace 2019, 21, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Sperzel, J.; Defaye, P.; Delnoy, P.P.; Garcia Guerrero, J.J.; Knops, R.E.; Tondo, C.; et al. Primary safety results from the LEADLESS Observational Study. Europace. 2018, 20, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Valiton, V.; Graf, D.; Pruvot, E.; Carroz, P.; Fromer, M.; Bisch, L.; et al. Leadless pacing using the transcatheter pacing system (Micra TPS) in the real world: Initial Swiss experience from the Romandie region. Europace 2019, 21, 275–280. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Al-Samadi, F.; Clementy, N.; Garweg, C.; Martinez-Sande, J.L.; Piccini, J.P.; et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018, 15, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Haeberlin, A.; Kozhuharov, N.; Knecht, S.; Tanner, H.; Schaer, B.; Noti, F.; et al. Leadless pacemaker implantation quality: Importance of the operator’s experience. Europace 2020, 22, 939–946. [Google Scholar] [CrossRef] [PubMed]
© 2023 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.