Introduction
Acute coronary syndrome (ACS) is a leading cause of morbidity and mortality in both men and women. There is growing evidence that female patients are underrepresented in clinical studies on ACS. This has led to a knowledge gap regarding the sex-specific differences in the presentation, pathophysiology, and outcomes of ACS in women [1]. Despite guidelines encouraging the inclusion of women in clinical trials, the representation of female patients remains low, with some studies reporting less than 30% female enrollment [2,3]. This lack of representation can result in underestimating the burden of disease and the impact of ACS in women. Furthermore, it may lead to suboptimal management of ACS in women, as treatments are often based on evidence derived from studies with predominantly male participants [1,4].
In recent years, there has been increasing awareness that the pathophysiology of ACS might be different in women then in men. Women have a higher prevalence of nonobstructive coronary artery disease, which is characterized by microvascular dysfunction, endothelial dysfunction, and coronary artery spasm. This contrasts men, who more often have obstructive coronary artery disease due to atherosclerotic plaque [5,6]. Regarding clinical profiles, women with ACS are often older and have a higher burden of comorbidities such as diabetes mellitus and chronic kidney disease, compared to men with ACS [7]. Hormonal differences are likely to play a central role in pathophysiology as estrogen has been shown to have cardioprotective effects in premenopausal women. However, the protective effects of estrogen are lost after menopause leading to a higher risk of ACS in postmenopausal women [8].
Among the different phenotypes of ACS, clinical entities such as takotsubo syndrome (TTS) and spontaneous coronary artery dissection (SCAD) have been increasingly reported as rare forms of ACS with a strikingly higher prevalence in women than in men. Despite the differences in the underlying pathophysiology, TTS and SCAD share other common clinical features, such as the acute onset with chest pain, electrocardiographic changes, and increase in cardiac biomarkers, besides the predominance in women. Both conditions are often anticipated by an emotional or physical stressful event considered as a trigger [9,10].
Despite the growing recognition and diagnosis of TTS and SCAD, many clinicians are still unfamiliar with these conditions leading to delayed diagnosis and suboptimal management. This review aims to provide a clinical overview of TTS and SCAD focusing on their epidemiology, clinical profiles, diagnostic workup, prognosis, and management, to enhance awareness and improve the management of these rare forms of ACS.
Definition and Epidemiology
Takotsubo Syndrome (TTS)
Takotsubo syndrome, also known as broken heart syndrome, stress-induced cardiomyopathy, or apical ballooning cardiomyopathy, is characterized by acute, transient left ventricular dysfunction in absence of epicardial coronary artery culprit lesions [9,11]. The pathophysiology of TTS is likely to be associated with acute catecholamine spillover following emotional or physical stressors leading to coronary microvascular dysfunction and direct myocardial damage [12]. In consideration of underlying pathophysiology, TTS has been previously defined as a microvascular form of ACS [13].
The condition was first described over three decades ago by Sato et al. in a report with five cases in a Japanese textbook. The term “takotsubo” is derived from a Japanese word meaning “octopus trap” because of the similarity of the end-systolic shape of the left ventricle in ventriculography with a narrow neck and apical ballooning [14].
Initial reports suggested that TTS exclusively affected individuals of Asian descents. However, over time the disorder has been increasingly recognized on a global scale. Currently, TTS is estimated to be diagnosed in about 2% of patients presenting with ACS, with rates increasing up to 8% in female patients [15,16]. The condition is known to affect mainly postmenopausal women, with up to 90% of patients with TTS being female. Nevertheless, with greater awareness of TTS more male patients are being diagnosed, particularly in cases anticipated by physical triggers [9,17].
In 2015, the International Takotsubo (InterTAK) Registry was established in Zurich encompassing the largest number of patients diagnosed with TTS and contributing significantly to the understanding of this still underrecognized condition [18].
Spontaneous Coronary Artery Dissection (SCAD)
Spontaneous coronary artery dissection is a rare form of ACS that is not caused by atherosclerosis, trauma, or medical intervention. This condition occurs when the layers of the coronary artery separate spontaneously due to either hemorrhage of the vasa vasorum or an intimal tear. This leads to the formation of a false lumen with an intramural hematoma, which can compress the coronary artery’s true lumen and cause myocardial infarction downstream [19]. The first case of SCAD was reported by Pretty in 1931, and for many years thereafter, the description of this condition has been limited to case reports [20]. However, in the last decade the diagnosis of SCAD has become more prevalent, likely due to the routine implementation of intracoronary imaging techniques such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT).
According to contemporary reports, SCAD accounts for approximately 4% of patients admitted with ACS and up to 35% of women under the age of 50 presenting with ACS [21,22]. Notably, SCAD is a leading cause of pregnancy-associated myocardial infarction (up to 43%) [23]. However, the true prevalence is likely still underestimated due to the challenge in establishing a definitive diagnosis. While over 90% of affected patients are women, SCAD has also increasingly been reported in men, who are usually younger than female patients [24].
Clinical Profiles and Diagnostic Work-up
Takotsubo Syndrome
Early diagnosis of TTS is challenging and requires implementation of several diagnostic tools, from cardiac catheterization to non-invasive multimodality imaging. Awareness of typical clinical characteristics of patients with TTS plays a critical role.
A major feature of TTS is the presence of an emotional or physical stressor preceding the onset of symptoms, identifiable in about 70% of cases [9,25]. Emotional triggers are commonly related to negative life events such as grief, panic, or interpersonal conflicts. However, joyful events have also been reported in association with a TTS onset. This peculiar condition is referred to as “happy heart” syndrome [26]. Physical triggers encompass a wide range of events including acute neurological disorders, physical activity, medical conditions, and iatrogenic factors such as administration of catecholamines. It is noteworthy that about one-third of patients with TTS do not have an identifiable trigger [25]. Emotional and physical triggers associated with TTS are illustrated in
Figure 1 [27].
Chronic psychiatric comorbidities, such as anxiety disorder or family history of psychiatric disease, have been assumed to represent predisposing factors for TTS, as patients with TTS have a higher prevalence of such conditions compared to the general population [9,28]. This association was confirmed in an ageand sex-matched comparison between patients with TTS and myocardial infarction demonstrating a higher prevalence of psychiatric and neurological disorders in the TTS group. The underlying mechanism of this correlation may involve increased adrenergic activation in response to stressors and impairment of norepinephrine reuptake in patients with chronic neuropsychiatric disorders [29].
Clinical presentation of TTS often resembles that of an acute myocardial infarction with a sudden onset of symptoms such as chest pain, dyspnea, or syncope. A second possible scenario concerns patients already hospitalized who develop TTS as a complication of a primary acute medical condition or treatment, considered as a physical trigger. In such cases, TTS can manifest with new onset of acute heart failure, arrhythmias, increase in cardiac biomarkers, and abnormalities in the electrocardiogram (ECG) [30,31].
A hallmark of TTS is the presence of transient left ventricular systolic dysfunction, with myocardial wall motion abnormalities extending beyond the vascular distribution of a single epicardial coronary artery, which usually resolves within a few days or weeks. According to the distribution of the wall’s motional abnormalities, different morphological forms of TTS have been described [32]. The most common anatomical variant is the apical type, also defined as apical ballooning or typical TTS, with a prevalence of about 82%. This form is characterized by hypo-, a-, or dyskinesis of all apical and midventricular myocardial segments with basal hypercontractility. Other anatomical variants, defined as atypical TTS, include midventricular, basal, and focal type (
Figure 2) [9]. Notably, the focal type is the only form with regional wall motion abnormalities that might correspond to the distributional territory of a single epicardial coronary artery. Right ventricular involvement, characterized by akinesia or dyskinesia of apical and/or midventricular segments and normal contraction of basal segments (“reverse McConnell’s sign”), occurs in approximately 15% of patients and is associated with adverse in-hospital outcomes [33].
The diagnosis of TTS traditionally relied on the Mayo Clinic diagnostic criteria first published in 2006 and revised in 2008 [34]. Over the years, other research groups have proposed their own diagnostic criteria. More recently, the InterTAK diagnostic criteria have been introduced including some novel aspects. These criteria particularly recognize pheochromocytoma as a physical trigger for TTS, whereas it was previously considered an exclusion criterion [25]. Additionally, InterTAK criteria acknowledge that a consistent number of patients with TTS might have a bystander coronary artery disease. In this regard, a recent study reported a prevalence of obstructive coronary artery disease in TTS of up to 23% [35].
Electrocardiogram
The ECG of patients with TTS often shows abnormal findings such as ST-segment elevation or T-wave inversion. In rare cases, left bundle brunch block, ST-segment depression, or even a normal finding has been reported on admission [9]. ECG abnormalities usually show a dynamic progression with resolution of the initial ST-segment abnormalities, followed by a progressive, prominent T-wave inversion and QT-interval prolongation. In some cases, T-wave inversion is detected on admission ECG, likely reflecting a delayed presentation. Prolongation of the QT-interval has been reported in association with ventricular arrhythmias including torsades de pointes and ventricular fibrillation [36].
A recent study identified specific ECG criteria which could differentiate between TTS and acute myocardial infarction. However, despite typical features, this differential diagnosis cannot exclusively rely on ECG [37].
InterTAK Diagnostic Score
A diagnostic tool known as the InterTAK Diagnostic Score has been established by comparing 218 patients with TTS and 436 patients with acute myocardial infarction or unstable angina. The score is based on easily obtainable parameters such as gender, trigger type, presence of ST-segment depression, psychiatric and neurological disorders, and QT-interval prolongation. The total score ranges from 0 to 100, with a score of ≤30 indicating a probability of less than 1% for TTS, and a score of >70 indicating a probability of TTS of about 90%. This tool has the potential to estimate the likelihood of TTS in the emergency department before the implementation of imaging modalities [38].
Biomarkers
Serum biomarkers of cardiomyocyte injury such as troponin, creatine kinase, and creatine kinase-MB are typically elevated in patients with TTS. However, peak levels are lower than in acute myocardial infarction and not proportional to the wide extent of myocardial wall motion abnormalities [9]. Conversely, patients with TTS often present high levels of B-type natriuretic peptide and N-terminal prohormone of BNP, which reach their peak 24–48 h after onset and are usually higher than in patients with myocardial infarction [39].
Cardiac Catheterization
Coronary angiography is considered the gold standard for ruling out myocardial infarction in patients with suspected TTS. The timing of the procedure should be determined according to the current guidelines for the management of ACS [30].
Biplane left ventriculography plays a crucial role in the diagnostic work-up, as it may reveal typical patterns of wall motion abnormalities and increase suspicion of TTS. The “apical nipple” sign is defined as a small area of the left ventricular apex with preserved contractility and has been reported in about 30% of patients with TTS. This sign can be useful to discriminate TTS from an anterior myocardial infarction [40]. In addition, ventriculography may be useful to rule out mechanical complications such as mitral regurgitation, left ventricular thrombi, or ventricular rupture. Slow pullback of a pigtail catheter from the left ventricular cavity allows to rule out a left ventricular outflow obstruction (LVOTO) [41].
Non-Invasive Multimodality Imaging
Transthoracic echocardiography is the firstline imaging modality in stable patients presenting with non-ST-elevation ACS. Echocardiography allows assessment of the left ventricular ejection fraction, detection of wall motion abnormalities distribution, and identification of TTS morphological variants. In particular, the circumferential pattern of wall motion abnormalities is considered a hallmark of TTS, especially for the apical and midventricular anatomical variants. Furthermore, echocardiography is useful in detecting right ventricular involvement and mechanical complication such as LVOTO, significant mitral regurgitation, and left ventricular thrombi [42]. Echocardiography is also important for confirming complete recovery with normalization of wall motion abnormalities and left ventricular ejection fraction during follow-up [42,43].
Cardiac magnetic resonance imaging (CMR) allows multiparametric tissue characterization and represents an essential tool in the diagnostic work-up of TTS. Typical findings on CMR include detection of regional wall motion abnormalities, presence of myocardial edema in T2-weighted black blood sequences, and absence of late gadolinium enhancement (LGE), which is useful in differentiating TTS from myocarditis and myocardial infarction with non-obstructive coronary arteries (MINOCA) [44]. Patchy LGE can be detected in a consistent number of patients with TTS when using LGE signal intensity of three standard deviations. To rule out any differential diagnosis other than TTS, it is recommended to use an LGE signal intensity threshold of five standard deviations, as no LGE is detectable in TTS [45].
Cardiac computer tomography angiography (CCTA) may be considered instead of coronary angiography to rule out relevant coronary artery disease in selected stable patients with elevated pretest probability of TTS or in critically ill patients [36].
Spontaneous Coronary Artery Dissection
The interplay between precipitating and predisposing factors is crucial in the pathogenesis of SCAD. Acute stressors have been reported to anticipate symptom onset in a consistent proportion of patients [10]. Most patients with an identifiable trigger experience intense emotional stressors such as death in the family, stress at work, relationship breakdown, or intense argument. In a previous study, Saw et al. described the prevalence of different precipitating factors preceding SCAD [46]. Overall, an emotional trigger was identified in about 40% of patients. Physical triggers related to intense physical activity, particularly isometric exercises, were reported in about 24% of patients, while other Valsalva-like activities such as vomiting, bowel movement with straining, or intense coughing occurred in about 2% of cases [46]. It is important to note that up to 40% of patients with SCAD did not present any identifiable triggers [46].
It has been postulated that emotional and physical stressors may induce SCAD through distinct pathophysiological mechanisms with preexisting susceptibility as a contributing factor. Physical stressors and Valsalva maneuvers have been linked to increased thoracic and abdominal pressure, which in turn leads to an elevated cardiocirculatory shear stress and potentially to SCAD. Conversely, emotional stressors have been associated with an increased release of catecholamines resulting in augmented myocardial contractility, coronary vasospasm, and ultimately intimal tear or hemorrhage of the vasa vasorum [10].
The evidence that not all individuals exposed to emotional or physical stressors experience SCAD underlines the importance of predisposing factors. Fibromuscular dysplasia (FMD) has been proposed as a major risk factor for the development of SCAD. The prevalence of this disorder in patients with SCAD has been previously reported to range from 17 to 86%, depending on the imaging techniques used for screening and the patient population studied [21,46,47]. FMD affects the smooth muscle and connective tissue of arteries leading to histological changes that increase susceptibility to developing stenosis, aneurysm, or dissection [48]. Notably, the higher prevalence of coronary tortuosity in patients with SCAD raised the hypothesis that the condition could be an expression of coronary FMD and predispose individuals to SCAD [21,49]. Inherited arteriopathies and connective tissue disorders such as Marfan syndrome, LoeysDietz syndrome, and Ehlers-Danlos syndromes have been identified as possible predisposing factors for SCAD. However, their prevalence in patients with SCAD is low (1–3%) [21,46,50].
Correlation between SCAD and systemic inflammatory conditions has been described in case reports and may be attributed to coronary artery vasculitis. In a previous study, Saw et al. observed that systemic inflammatory conditions were present in approximately 8.9% of patients with SCAD. In contrast, Alfonso et al. screened 27 patients with SCAD for markers of inflammation, and none of them showed any signs of inflammatory disease [46,51]. It is plausible that a small percentage of patients with SCAD have underlying inflammatory conditions, but this relationship has yet to be definitively established.
Hormonal factors, specifically related to pregnancy and menopause, have been proposed as important contributors to the higher incidence of SCAD in women. Pregnancy is estimated to be associated with 2–8% of SCAD cases, with the correlation being likely multifactorial [52]. Estrogen exposure may induce alterations in collagen tissues and weaken arterial walls, while the increase in circulatory volume and intraabdominal pressure during pregnancy can lead to elevated vascular shear stress [10].
Among comorbidities, patients with SCAD exhibit a higher prevalence of psychological distress, anxiety, and depression, compared to patients with non-SCAD ACS [21].
Differential diagnosis for SCAD includes conditions such as atherosclerotic ACS, TTS, coronary spasm, and MINOCA. Clinical presentation is unspecific and does not allow differentiation of SCAD from these conditions. The most frequent presenting symptoms is chest pain occurring in about 96% of patients with onset of SCAD [53]. Less frequently, patients experience symptoms such as radiation to the arm or neck, nausea or vomiting, diaphoresis, and dyspnea [53]. Different studies have reported that on admission ECG, ST-elevation is observed in 26 to 55% of cases, while non-ST-elevation is observed in 13 to 69% of cases [54,55,56,57]. Although cardiac enzymes are usually elevated, a recent study based on a Japanese cohort demonstrated that mean peak creatinine kinase level was lower in SCAD as compared with non-SCAD ACS [56].
Cardiac Catheterization
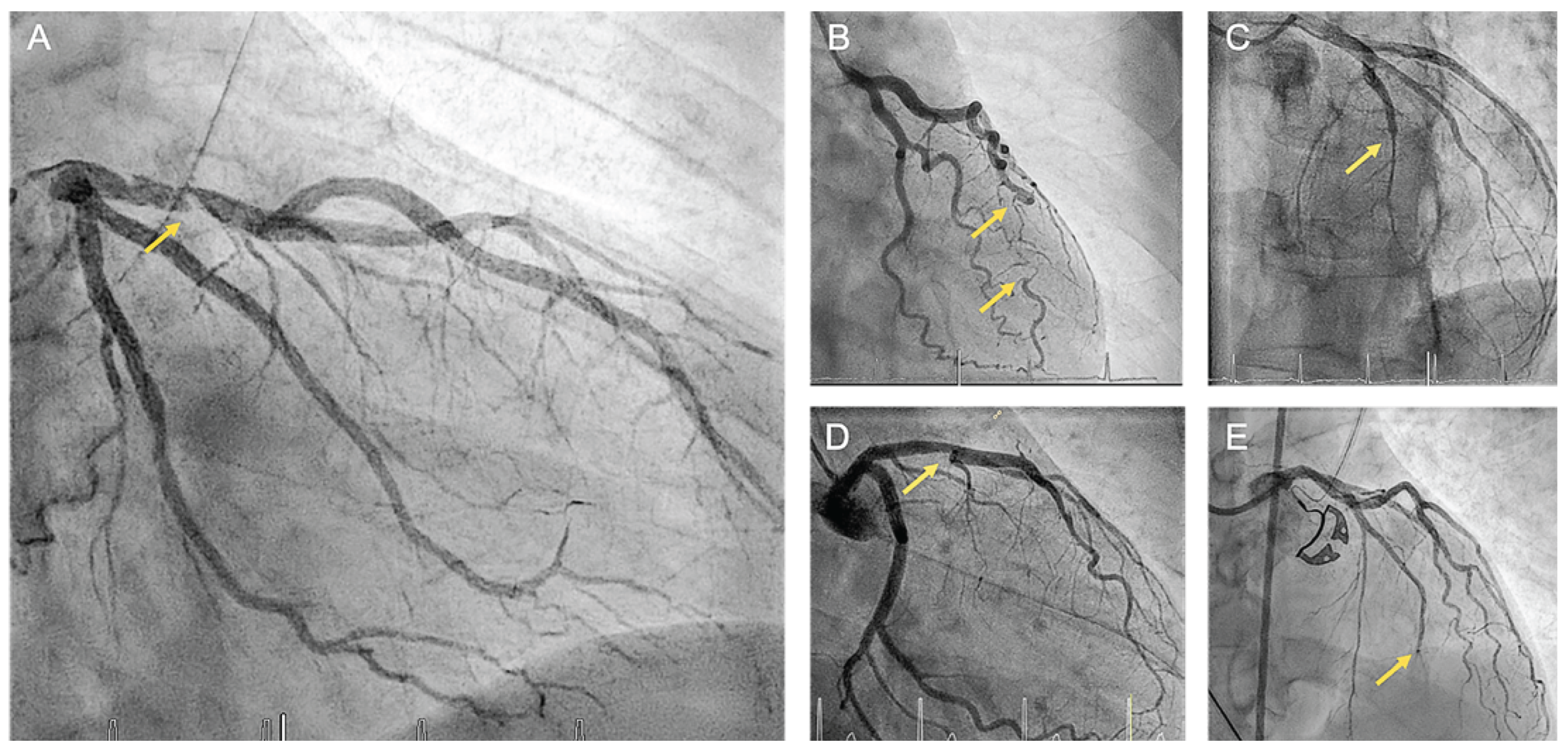
Early and accurate diagnosis of SCAD is imperative as the management differs significantly from atherosclerotic myocardial infarction. For patients presenting with ACS or suspected SCAD, the first-line investigation is typically coronary angiography, which allows the categorization of SCAD into distinct angiographic types (
Figure 3). SCAD type 1 is characterized by contrast dye staining of the arterial wall and multiple radiolucent lumens. SCAD type 2 refers to a diffuse stenosis resulting from an abrupt change in arterial caliber with variable length and severity, typically exceeding 20 mm. This can be bordered by normal artery segments proximal and distal to the intramural hematoma (type 2a) or extend to the distal tip of the artery (type 2b). SCAD type 3 presents with focal or tubular stenosis typically <20 mm in length mimicking atherosclerosis. Among the different angiographic types, SCAD type 2 is the most frequent (approximately 68%), followed by type 1 (approximately 29%) and type 3 (approximately 3%) [19,57,58]. More recently, a new variant of SCAD referred to as type 4 has been proposed, which is characterized by total occlusion usually affecting a distal vessel. Diagnosis of this rare form is challenging and requires reestablishment of blood flow through coronary intervention. Furthermore, it is necessary to exclude sources of coronary embolism [59].
Intracoronary Imaging
The implementation of intracoronary imaging facilitates the detection of intramural hematoma or a double lumen. Both IVUS and OCT have proven to being useful in the diagnosis of SCAD. The selection of the imaging technology depends on the operator’s preference and its availability. IVUS provides superior depth penetration and is effective in assessing the extent of intramural hematoma without the need for contrast injection. It generally allows visualization of intimal tear and false lumen, however, its limited spatial resolution (approximately 150 µm) may impede the identification of small structures related to SCAD [60,61]. In contrast, OCT provides higher spatial resolution (10–20 µm), enabling detailed characterization of the true and false lumen and improved delineation of the lumen-intimal interface [62]. Consequently, OCT is usually considered as the preferred imaging modality in SCAD diagnostic work-up. It is important to acknowledge that utilization of intracoronary imaging in the context of SCAD is not without risk. Potential complications such as dissection extension with the wire or diagnostic catheter, hydraulic dissection extension because of contrast injection during OCT, and catheter-induced occlusion of the true lumen, must be considered before its implementation [21,63]. Therefore, the use of intracoronary imaging should be reserved for situations when the coronary angiographic diagnosis is uncertain, the vessel diameter is favorable and only after careful consideration of risks and benefits.
Cardiac Computer Tomography Angiography
CCTA is gaining importance for evaluating coronary arteries in low-to-intermediate risk patients with chest pain [64]. Data supporting its use in the diagnostic work-up of SCAD are scarce. Compared to invasive angiography, CCTA has the advantage of allowing coronary assessment without the increased risk of an iatrogenic dissection. However, the lower spatial resolution is a limitation for accurate assessment and interpretation of the smaller mid-todistal coronary territories, which are more prone to SCAD [65]. Also, false negative findings have been reported [57,66]. Therefore, coronary angiography remains the recommended primary diagnostic investigation in patients suspected of having SCAD. However, CCTA can play a role in the follow-up assessment of SCAD affecting proximal and largecaliber vessels [21].
Prognosis and Clinical Outcomes
Takotsubo Syndrome
TTS was traditionally considered a benign condition due to the reversible nature of the systolic dysfunction. However, recent research suggests that a significant number of patients may experience serious complications and that the rate of in-hospital mortality is comparable to patients with myocardial infarction or unstable angina (1–4.5%) [9]. Adverse events are primarily caused by the sudden impairment of left ventricular function leading to acute heart failure in 12 to 45% of cases, and cardiogenic shock in 6 to 20% of cases [36,67]. Hemodynamic deterioration can also be induced by LVOTO, which is detected in 7 to 20% of patients and is often associated with systolic anterior motion of the mitral valve leaflets and significant mitral regurgitation [41].
During the hospital stay, 2–10% of patients experience ventricular arrhythmias, such as ventricular tachycardia, torsades de pointes, and ventricular fibrillation. In about 5% of cases, there is a link between TTS and cardiac arrest [68,69]. Approximately 3% of TTS patients have been found to have intraventricular thrombi and/or cardioembolic complications [70]. Additionally, rare but life-threatening complications include ventricular septal defect and left ventricular free wall rupture occurring in less than 1% of cases [36].
The long-term outcomes of TTS have been less extensively investigated but received increased research attention over the last years. Elesber et al. were among the first to report on this issue and found that the four-year mortality rate among 100 patients with TTS was comparable to that of ageand gender-matched individuals without TTS [71]. More recently, Tornvall et al. found that the mortality rate in TTS was higher than in control subjects without coronary artery disease, and comparable to that of patients with coronary artery disease at long-term follow-up [72]. This suggests that prognosis of TTS may be worse than initially thought. In the InterTAK cohort, TTS patients demonstrated comparable long-term outcomes when compared to ageand sexmatched patients with myocardial infarction or unstable angina [73]. Notably, TTS patients with emotional triggers or no identifiable trigger exhibited significantly better outcomes than those with acute myocardial infarction or unstable angina. Conversely, patients with physical triggers had a higher long-term mortality compared to those with acute myocardial infarction or unstable angina [73]. These findings suggest that the burden of comorbidities may be an important determinant of the prognosis of patients with TTS.
Finally, recurrence of TTS is an important long-term complication, occurring in about 5% of patients with an incidence rate of 18.7 cases per 1,000 patient-years [74].
Spontaneous Coronary Artery Dissection
SCAD is usually associated with low rates of in-hospital mortality, ranging from 0 to approximately 2% across different studies [10,51,75,76]. During the acute phase, patients may experience major complications such as ventricular arrhythmias, with a prevalence of up to 10%. Recurrent in-hospital myocardial infarction has been reported in up to 5% of cases and is often related to the extension of the index dissection. Additionally, cardiogenic shock has been reported in up to 3% of patients [46,54,55,76]. In a previous study, Hill et al. described sudden cardiac death in less than 1% of patients with SCAD, however, this data might be underestimated [10].
A substantial number of patients with SCAD suffer major cardiac adverse events (MACE) after discharge. MACE have been reported in 10-20% of patients at the two-year follow-up and up to 50% at the ten-year follow-up [46,52,55,56]. Despite favorable longterm survival rates, recurrent SCAD represents a major long-term complication, which occurs in up to 30% of patients at fourto tenyear follow-up [46,52,55,56]. Therefore, identifying high-risk patients becomes crucial. In this regard, Eleid et al. described a correlation between coronary tortuosity and recurrence of SCAD [49].
Recently, Saw et al. reported genetic disorders, peripartum status, and presence of extracoronary FMD, to be independently associated with increased risk of MACE at three-year follow-up [76].
Clinical Management
Specific recommendation for management of TTS and SCAD are lacking as no prospective randomized clinical trials have been performed. Current therapeutic strategies are therefore based on case series and expert consensus.
Takotsubo Syndrome
The in-hospital management of patients with TTS should be focused on supportive care to minimize complications until recovery. In a previous study, Lyon et al. proposed a stratification system to identify patients at higher risk for in-hospital complications [30]. With the same purpose, Santoro et al. recently established the GEIST (German and Italian Stress Cardiomyopathy) prognostic score [77]. Such tools may be useful for in-hospital risk stratification and to guide patient management.
Patients with severe hypotension or cardiogenic shock should undergo prompt rule-out of LVOTO with Doppler echocardiography or invasively at time of cardiac catheterization. In patients with TTS complicated by LVOTO, positive inotropic agents should be discontinued as they may enhance basal hypercontractility and increase the intraventricular gradient [41]. Diuretics should be used with extreme caution and only in case of pulmonary oedema since diuresis may worsen LVOTO through a reduction of preload. Conversely, careful fluid administration and application of short half-life beta1-selective beta blockers such as esmolol or landiolol may improve preload and resolve the intraventricular gradient [41,78].
In patients who are hemodynamically unstable but do not have LVOTO, administration of positive inotropic agents can be necessary. However, the use of catecholamines in the context of TTS has been a subject of controversy due to safety concerns. Recent studies have proposed that levosimendan, a Ca2+-sensitizer, could be a viable and secure alternative to catecholamines [79]. Alternatively, early implementation of mechanical circulatory support with devices such microaxial blood pump catheter could be considered. For patients who are experiencing refractory cardiogenic shock, venoarterial extracorporeal membrane oxygenation can be considered as a last resort [36].
Patients with congestive heart failure who are hemodynamically stable may benefit from diuretics or nitroglycerine to reduce preload, while there is no evidence of the prognostic benefit of medications such as angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blocker (ARB) inhibitors or beta blockers in the acute phase [80].
Due to consistent risk of left ventricular thrombus and systemic embolism, the use of anticoagulation can be considered on a patient basis. Expert consensus suggests that patients with left ventricular ejection fraction <30% and large apical involvement could benefit from anticoagulation until improvement of the wall motion abnormalities, in absence of a relevant bleeding risk [30,36,70].
In consideration of the high prevalence of QT-interval prolongation with associated increased risk of ventricular arrhythmias, patients with TTS should undergo ECGmonitoring for at least 24 h and until normalization of QT-interval. Owing to the transient nature of cardiac dysfunction, use of implantable cardiovert defibrillator is of uncertain benefit both in primary and secondary prevention. However, wearable defibrillators (life vest) could be considered in selected cases [36].
Long-term therapy of TTS is not well established. There have been several studies that have investigated the use of beta blockers and ACE inhibitors in the management of TTS, but the results have been controversial and thus require further validation [80]. The use of statin and acetylsalicylic acid should be personalized and based on the presence of comorbidities such as coronary artery disease. Given that neuropsychiatric disorders have been considered potential predisposing factors, some patients with TTS may benefit from psycho-cardiological rehabilitation and specialist counseling [36].
Spontaneous Coronary Artery Dissection
It is commonly observed that most cases of SCAD undergo spontaneous healing. Furthermore, compared to atherosclerotic ACS, percutaneous coronary intervention (PCI) procedures are associated with higher rate of complications, failure rates, and worse outcomes in patients with SCAD [46,54,56]. Therefore, a conservative approach is generally preferred where revascularization is not urgently indicated. In this regard, patient selection is crucial and an individualized decision should be made based on clinical and angiographic parameters [19]. Patients with persistent ischemia or hemodynamic instability, obstructive SCAD with reduced coronary flow, or high-risk anatomy (involvement of left main, proximal left anterior descendent or circumflex or right coronary artery, multivessel SCAD), should be considered for revascularization [19]. The decision between PCI and coronary artery bypass grafting should be based on the patient’s anatomy, characteristics, and the local expertise. Noteworthy, an extended inpatient monitoring for up to five days is recommended for conservatively managed SCAD, as in most of cases, failure of conservative management occurs during early follow-up [10].
Patients undergoing PCI with stent implantation receive dual antiplatelet therapy (DAPT) followed by antiplatelet monotherapy according to current ACS guidelines [19]. Nevertheless, the role of antiplatelet therapy in patients with conservatively managed SCAD is less established due to safety concerns related to the pathophysiology of the condition with spontaneous intramural hematoma. Previous studies suggested that intimal tear can represent a substrate for thrombus formation in the true lumen. Therefore, antiplatelet therapy with acetylsalicylic acid is usually recommended in the acute phase and many authors recommend the use of dual antiplatelet therapy with additional clopidogrel [10,21,81]. The optimal duration of DAPT is unknown and whether subsequent acetylsalicylic acid monotherapy should be continued lifelong is a matter of discussion.
Although anticoagulation is commonly administered to patients with ACS prior to and during coronary angiography with PCI, there is currently no evidence regarding the risks and benefits of anticoagulation in SCAD. Due to the potential risk of extending dissection, anticoagulation is usually discontinued at the time of diagnosis of SCAD in the absence of other strong indications [59].
Hypertension has emerged as a potential predictor of SCAD recurrence. Therefore, optimal blood pressure control is recommended for patients with SCAD. Therapy with beta blockers has been recently linked to a reduced rate of recurrence and is generally recommended as first-line therapy for both acute and long-term management [19,82]. The use of other medications such as ACE or ARB inhibitors, mineralocorticoid receptor antagonists, or gliflozins has not been extensively studied regarding SCAD and should be considered in patients with reduced left ventricular function according to current guidelines for management of patients with heart failure [10].
Pregnancy-associated SCAD requires special consideration and should be managed by a multidisciplinary team with expertise in cardiovascular and gynecological-obstetric care. The management of this subgroup of patients should follow the usual recommendations for SCAD management but with a stronger preference for a conservative approach, avoidance of teratogenic drugs, and minimization of exposure to ionizing radiation [21].
In consideration of the high prevalence of non-coronary arteriopathies, expert consensus recommends a vascular screening after the diagnosis of SCAD. Saw et al. proposed a comprehensive diagnostic approach, which includes non-selective angiography of the iliac and renal arteries at the time of index coronary angiography to detect FMD, as well as a computed tomography angiography (CTA) of the neck and head to exclude cerebrovascular FMD and intracranial aneurysms [10]. A recent position paper proposed a less invasive approach, involving the implementation of a CTA protocol from head to pelvis [57]. Alternatively, despite a lower spatial resolution, magnetic resonance angiography represents a radiation-free option for selected patients [57]. Follow-up management of patients with SCAD includes assessment of left ventricular function with echocardiography.
Differences and Similarities
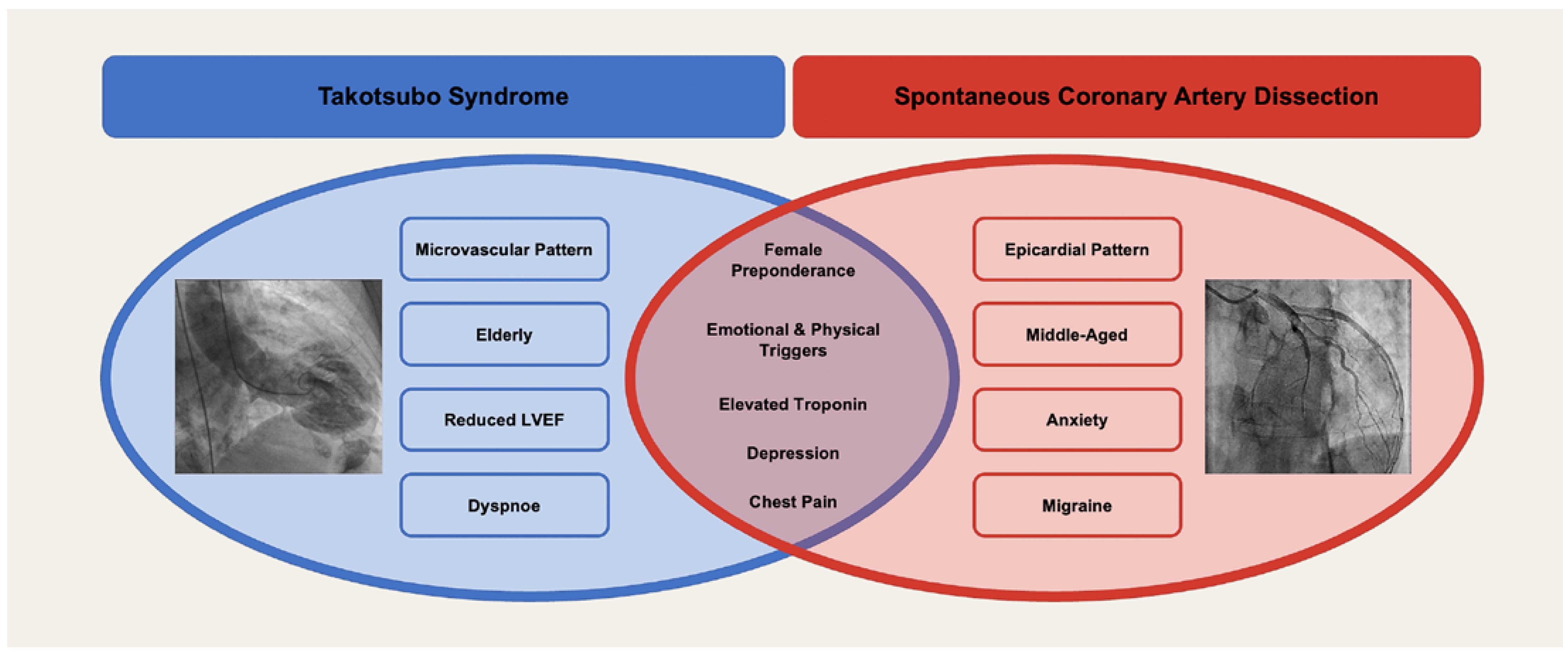
TTS and SCAD are two distinct entities that share many similarities (
Figure 4) [83]. Both conditions are an important differential diagnosis of ACS with a predominance in women. While TTS more often affects postmenopausal women, SCAD is more prevalent in younger women. On average, a SCAD patient is about twenty years younger than a TTS patient. Both TTS and SCAD are typically anticipated by emotional or physical triggering factors and present with similar symptoms such as chest pain, shortness of breath, dizziness, sweating, syncope, subsequently associated with changes in ECG and cardiac biomarkers.
While physical triggers are more common in TTS, emotional triggers tend to be more prevalent in SCAD [83]. Both SCAD and TTS are often accompanied by neuropsychiatric comorbidities. However, their relevance in the development of these two conditions remains to be conclusively determinated.
Patients with SCAD generally exhibit a higher incidence of comorbidities such as migraine and anxiety, whereas patients with TTS often present a greater burden of cardiovascular risk factors [83].
The pathophysiology of TTS and SCAD is certainly different. Whereas TTS is most probably a microvascular disorder, SCAD affects the epicardial coronary arteries. Both TTS and SCAD can show a wide range of outcomes, from being benign and reversible to being associated with life-threatening complications including death. Research in the field is continuing and of utmost importance to understand the mechanisms and pathophysiology of TTS and SCAD and to allow researchers and clinicians to develop therapeutic targets and preventive strategies.
 You will find the full list of references online at https://cardiovascmed.ch/article/doi/ CVM.2023.1246945772.
You will find the full list of references online at https://cardiovascmed.ch/article/doi/ CVM.2023.1246945772.