How to Assess Bleeding Risk in Patients Undergoing Percutaneous Coronary Interventions
Abstract
Background and Aim
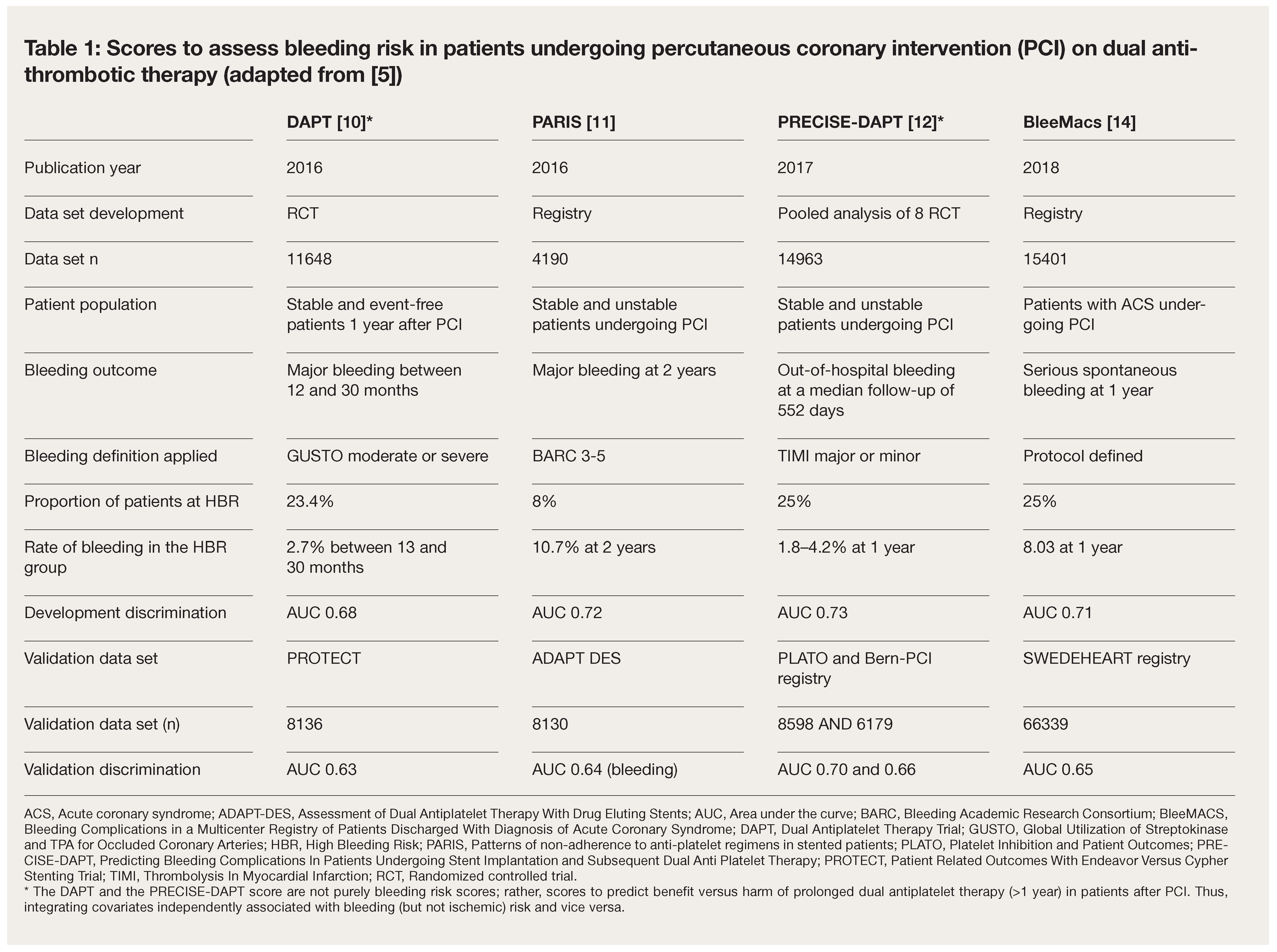
Underrepresentation of the High Bleeding Risk PCI Group in Previous Scores
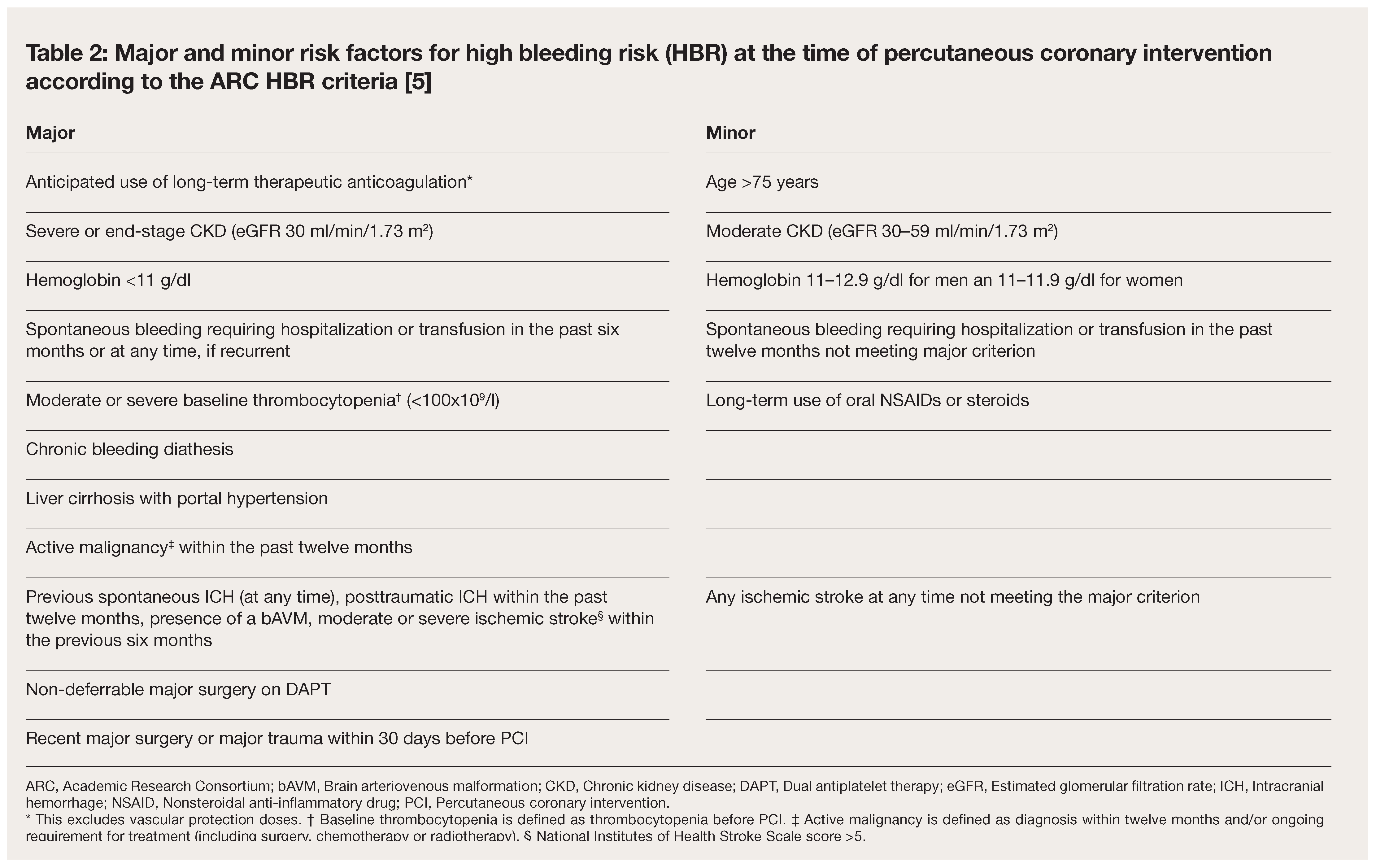
Standardization of Criteria for High Bleeding Risk (HBR) Based on HBR PCI Population
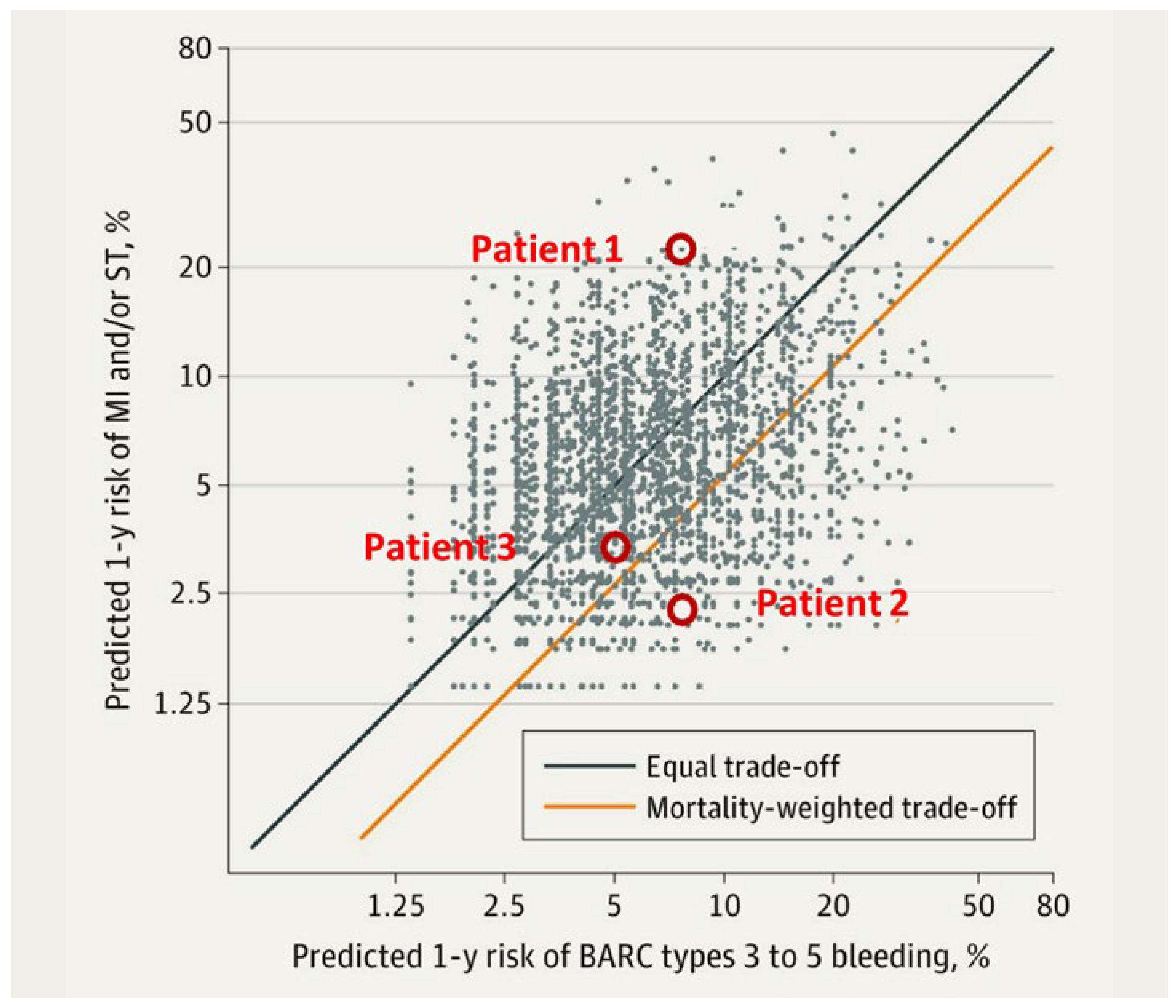
The Arc-HBR Trade O Model [6]: A Two-Step Risk Stratification Approach to Individualize the Antithrombotic Management in PCI Patients with HBR
| BARC type 3–5 bleeding | MI and/or ST | |||
|---|---|---|---|---|
| Predictor | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age > 65 | 1.50 (1.08–2.08) | 0.01 | NA | NA |
| Diabetes | NA | NA | 1.56 (1.26–1.93) | <0.001 |
| Prior MI | NA | NA | 1.89 (1.52–2.35) | <0.001 |
| Liver disease, cancer or surgery | 1.63 (1.27–2.09) | 0.0001 | NA | NA |
| COPD | 1.39 (1.05–1.83) | 0.02 | NA | NA |
| Current smoker | 1.47 (1.08–1.99) | 0.01 | 1.48 (1.09–2.01) | 0.009 |
| NSTEMI or STEMI presentation | NA | NA | 1.82 (1.46–2.25) | <0.001 |
| Haemoglobin, g/dl | ||||
| >13 | 1 (Reference) | 1 (Reference) | ||
| 11–12,9 | 1.69 (1.30–2.20) | <0.001 | 1.27 (.99–1.63) | 0.005 |
| <11 | 3.99 (3.06–5.20) | 1.50 (1.12–1.99) | ||
| eGFR, ml/min/1.73 m2 | ||||
| >60 | 1 (Reference) | 1 (Reference) | ||
| 30–59 | 0.99 (0.79–1.24) | 0.02 | 1.30 (1.03–1.66) | 0.001 |
| <30 | 1.43 (1.04–1.96) | 1.69 (1.20–2.37) | ||
| Complex procedureb | 1.32 (1.07–1.61) | 0.008 | 1.50 (1.21–1.85) | <0.001 |
| Bare metal stentc | NA | NA | 1.53 (1.23–1.89) | <0.001 |
| OAC at discharge | 2.00 (1.62–2.48) | <0.001 | NA | NA |
| C statistic | 0.68 | NA | 0.69 | NA |
Keypoints
- Approximately 40% of an all-comers percutaneous coronary intervention population meet the criteria for high post-interventional bleeding risk (HBR) with dual antiplatelet therapy.
- The balance between bleeding and thrombotic risk varies from HBR patient to HBR patient.
- In contrast to previous scores, a currently available model with stepwise app-based scoring (ARC HBR App) for bleeding and ischemia is based on a HBR population.
- This scoring should be applied individually to patients at high risk of bleeding and can contribute to improved risk stratification.
Conflicts of Interest
References
- Khan, S.U.; Singh, M.; Valavoor, S.; Khan, M.U.; Lone, A.N.; Khan, M.Z.; et al. Dual Antiplatelet Therapy After Percutaneous Coronary Intervention and Drug-Eluting Stents: A Systematic Review and Network Meta-Analysis. Circulation 2020, 142, 1425–1436. [Google Scholar] [CrossRef]
- Giustino, G.; Chieffo, A.; Palmerini, T.; Valgimigli, M.; Feres, F.; Abizaid, A.; et al. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J. Am. Coll. Cardiol. 2016, 68, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; ESC Scientific Document Group; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016, 134, e123–155. [Google Scholar]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 2019, 40, 2632–2653. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Gregson, J.; Owen, R.; Mehran, R.; Windecker, S.; Valgimigli, M.; et al. Assessing the Risks of Bleeding vs Thrombotic Events in Patients at High Bleeding Risk After Coronary Stent Implantation: The ARC-High Bleeding Risk Trade-off Model. JAMA Cardiol. 2021, 6, 410–419. [Google Scholar] [CrossRef]
- Dangas, G.D.; Claessen, B.E.; Mehran, R.; Xu, K.; Fahy, M.; Parise, H.; et al. Development and validation of a stent thrombosis risk score in patients with acute coronary syndromes. JACC Cardiovasc. Interv. 2012, 5, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Subherwal, S.; Bach, R.G.; Chen, A.Y.; Gage, B.F.; Rao, S.V.; Newby, L.K.; et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: The CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009, 119, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Pocock, S.J.; Nikolsky, E.; Clayton, T.; Dangas, G.D.; Kirtane, A.J.; et al. A risk score to predict bleeding in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 2010, 55, 2556–2566. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.W.; Secemsky, E.A.; Kereiakes, D.J.; Normand, S.L.; Gershlick, A.H.; Cohen, D.J.; DAPT Study Investigators; et al. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA 2016, 315, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Mehran, R.; Giustino, G.; Cohen, D.J.; Henry, T.D.; Sartori, S.; et al. Coronary Thrombosis and Major Bleeding After PCI With Drug-Eluting Stents: Risk Scores From PARIS. J. Am. Coll. Cardiol. 2016, 67, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; PRECISE-DAPT Study Investigators; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRE-CISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, G.; Wallace, J.S.; Baron, G.; Ravaud, P.; Alberts, M.J.; Wilson, P.W.; REACH Investigators; et al. Risk score to predict serious bleeding in stable outpatients with or at risk of atherothrombosis. Eur. Heart J. 2010, 31, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Raposeiras-Roubín, S.; Faxén, J.; Íñiguez-Romo, A.; Henriques, J.P.; D’Ascenzo, F.; Saucedo, J.; et al. Development and external validation of a post-discharge bleeding risk score in patients with acute coronary syndrome: The BleeMACS score. Int. J. Cardiol. 2018, 254, 10–15. [Google Scholar] [CrossRef]
- Pencina, M.J.; Goldstein, B.A.; D’Agostino, R.B. Prediction Models—Development, Evaluation, and Clinical Application. N. Engl. J. Med. 2020, 382, 1583–1586. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Meredith, I.T.; Abizaid, A.; Pocock, S.J.; Carrié, D.; Naber, C.; LEADERS FREE Investigators; et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2015, 373, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Ariotti, S.; Adamo, M.; Costa, F.; Patialiakas, A.; Briguori, C.; Thury, A.; ZEUS Investigators; et al. Is Bare-Metal Stent Implantation Still Justifiable in High Bleeding Risk Patients Undergoing Percutaneous Coronary Intervention?: A Pre-Specified Analysis From the ZEUS Trial. JACC Cardiovasc. Interv. 2016, 9, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Varenne, O.; Cook, S.; Sideris, G.; Kedev, S.; Cuisset, T.; Carrié, D.; SENIOR Investigators; et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): A randomised single-blind trial. Lancet 2018, 391, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- ARC_HBR app.
- Giustino, G.; Baber, U.; Sartori, S.; Mehran, R.; Mastoris, I.; Kini, A.S.; et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: A systematic review and meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2015, 65, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
© 2023 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Rickli, H.; Maeder, M.T. How to Assess Bleeding Risk in Patients Undergoing Percutaneous Coronary Interventions. Cardiovasc. Med. 2023, 26, 111. https://doi.org/10.4414/cvm.2023.02270
Rickli H, Maeder MT. How to Assess Bleeding Risk in Patients Undergoing Percutaneous Coronary Interventions. Cardiovascular Medicine. 2023; 26(4):111. https://doi.org/10.4414/cvm.2023.02270
Chicago/Turabian StyleRickli, Hans, and Micha T. Maeder. 2023. "How to Assess Bleeding Risk in Patients Undergoing Percutaneous Coronary Interventions" Cardiovascular Medicine 26, no. 4: 111. https://doi.org/10.4414/cvm.2023.02270
APA StyleRickli, H., & Maeder, M. T. (2023). How to Assess Bleeding Risk in Patients Undergoing Percutaneous Coronary Interventions. Cardiovascular Medicine, 26(4), 111. https://doi.org/10.4414/cvm.2023.02270




