Heart failure: frequent and significant
Similar to other developed countries, cardiovascular mortality in Switzerland has gradually declined since the 1980s. This should however not obscure the fact that cardiovascular diseases are a major cause for hospitalisations. Between 2002 and 2018, the number of hospitalisations rose annually from 50,000 to 65,000 in men and from 45,000 to 50,000 in women [1]. Heart failure is particularly frequent in individuals aged 80 or older with an estimated prevalence of 20%. Roughly 200,000 people in Switzerland suffer from heart failure and yearly 18,000 die from it [2]. In the early stages, patients are usually compensated and have few symptoms and complaints, although the disease progresses. Once hospitalised for heart failure, one in four patients is rehospitalised within 30 days, with a substantially increased mortality risk after the second hospitalisation [3]. The 30-day in-hospital mortality rate of heart failure patients in Switzerland has remained stable at 8-8.5% since 2009, whereas for stroke, for example, it has declined by about 30% [2]. These figures illustrate that in heart failure it is particularly important to intervene early in the disease process and to influence its course and progression at a stage when this is still possible. One key element for the prevention of heart failure is blood pressure regulation: Since only too low, but not too high blood pressure is acutely life-threatening, the body initiates counter-regulatory mechanisms such as activation of the sympathetic stress axis and the renin-angiotensin-aldosterone system even at a slight decrease in cardiac output. The increased peripheral resistance and the raised blood volume place an additional burden on the weakened heart – the onset of a vicious circle (
Figure 1) [4].
Nutritional deficiencies can be a cause or a consequence
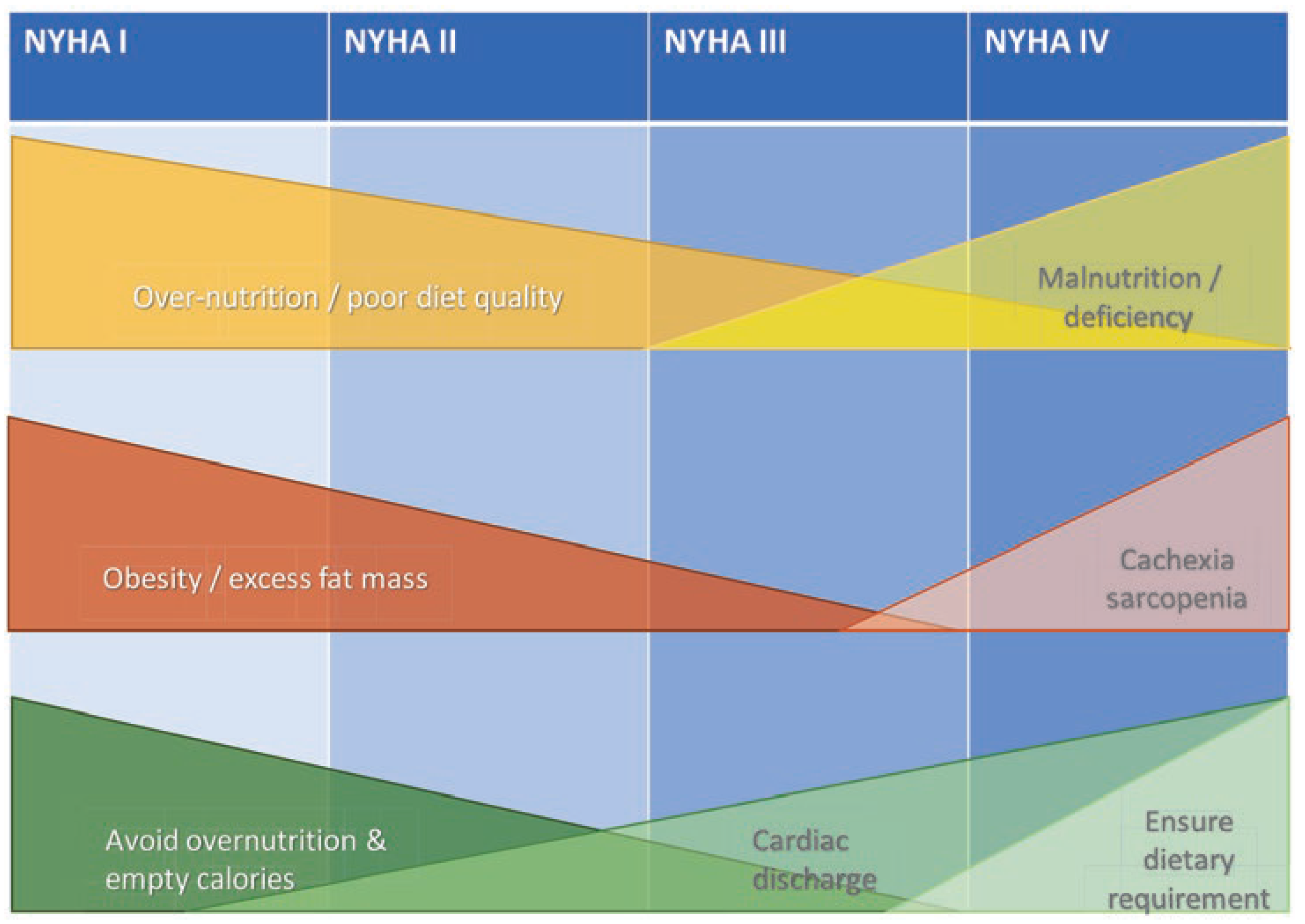
Cardiac rehabilitation after heart failure may reduce hospital admissions and confer a clinically important improvement in health-related quality of life [5]. Multidisciplinary rehabilitation includes exercise training, health behaviour change and education, lifestyle risk factor and long-term disease management [5,6]. Besides, treatment with testosterone has shown promising results [7]. Nutrition plays an important – yet underrecognised – role in the vicious cycle of heart failure development and progression. Particularly in the early stages of heart failure, dietary causes for its progression can be identified, such as a positive calorie balance, excessive consumption of processed foods high in salt and calories and insufficient consumption of fruits, vegetables, nuts and legumes [8]. In advanced stages, the disease increasingly influences dietary behaviour rather than vice versa. Here, malnutrition often takes centre stage. Its clinical presentation may vary from decrease of appetite and/or weight, to more isolated loss of muscle mass with sarcopenia, to cardiac cachexia, the latter including chronic inflammation, low testosterone levels and unintended weight loss. Deficiencies in energy-protein and micronutrients accelerate disease progression and further deteriorate heart function (
Figure 1 and
Figure 2). Contrary to their importance, nutritional recommendations for patients with heart failure are standing on a thin scientific foundation. The main reason for this is the lack of evidence coming from studies with limiting designs, small numbers of participants and usually short duration [4,9,10]. Another problem is that recommendations are formulated too generally and insufficiently consider individual circumstances, such as nutritional status and stage of heart failure. Measures that appear reasonable in early stages may be counterproductive in advanced stages. For example, salt reduction may make sense in the early stages of heart failure, whereas it may be counterproductive in advanced stages due to counterregulatory mechanisms. Moreover, it is difficult for patients to maintain a strict reduction of salt intake of, for example, less than 5 g per day [11]. Similarly, caloric restriction may improve cardiac function and support weight loss in older healthy individuals, whereas in advanced heart failure it may lead to deficiency and unintentional weight loss, which is associated with increased mortality [12–14]. The goal of nutritional interventions should be to prevent or at least mitigate progression of the vicious cycle as early as possible. In advanced stages, interventions must aim at preventing or correcting deficiency symptoms, enabling to improve quality of life and reduce the risk of rehospitalisation, morbidity and mortality.
Dietary interventions in heart failure patients
While diets rich in fried and animal products are associated with increased risk of heart failure, there are also diets that are associated with reduced risks of cardiovascular disease including heart failure. Several studies suggest that a plant-based diet with low processing degree may be heart failure-protective [8]. Indeed, there is convincing evidence from randomised controlled trials and from observational studies that a Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) dietary pattern can reduce the risk of heart failure in the general population [9,15]. In contrast, there are relatively few studies that have examined outcomes in heart failure patients. Some (but not all) of the trials with relatively small numbers of participants were able to find reduced mortality or rehospitalisation in patients with high adherence to the above-mentioned dietary patterns. Other trials demonstrated a reduction in blood pressure and improvements in cardiac parameters and the six minute walk test (6MWT) [9,10].
Especially in advanced stages, nutritional deficiencies become more prevalent in heart failure patients (see
Table 1). However, trials investigating the effects of supplementing patients with multivitamins or single nutrients have shown inconsistent results. Consequently, there are only a few strong recommendations in regard for the heart failure patient population (see
Table 2). Trials using multivitamin supplements and lasting less than twelve months showed little (approximately 5%) or no significant increase in left ventricular ejection fraction [16]. Intervention trials with vitamin D in heart failure patients with a deficiency, failed to find improvement in clinical outcomes or mortality [17]. Results are similar for other supplements: for example, intervention studies with coenzyme Q10 (CoQ10) demonstrated a decrease in cardiovascular incidence and mortality and hospitalisation, but no improvement in 6MWT or New York Heart Association functional classification (NYHA)[18,19]. A meta-analysis of over 80,000 heart failure patients did not find a significant effect of n-3 fatty acids on heart failure hospitalisations and cardiovascular mortality and only a small yet significant effect on rehospitalisations [20]. Studies with supplementation of protein and amino acids in heart failure patients showed that lean body mass and 6MWT could be improved – however without demonstrating an increase in muscle strength [21].
The most sound evidence of benefits for various endpoints in heart failure patients exists for intravenous iron administration – oral administration has had little success but has been insufficiently studied overall [22]. In fact, several trials have shown that intravenous iron therapy significantly improved several outcomes, including different cardiac parameters, quality of life, hospitalisation and mortality [23,24]. About half of the heart failure patients have too low levels of available iron. Therapy with iron is appropriate in anaemic patients with absolute iron deficiency. It remains unclear whether non-anaemic heart failure patients with absolute or functional iron deficiency should be treated, as long-term clinical endpoints, such as rehospitalisation and morbidity/mortality are still lacking [22].
Nutritional interventions in hospitalised patients
Heart failure patients who require hospitalisation often have a poor prognosis, particularly if patients are elderly and polymorbid. In the population of chronic heart failure patients, between 10% and 40% of patients are malnourished. This may be explained by different risk factors including old age, high burden of comorbidities, intestinal oedema leading to malabsorption, inflammation with elevated cytokines causing loss of appetite and anorexia and fatigue with dyspnoea leading to impairment in activities of daily living. In addition, once admitted to the hospital, these patients are at high risk for further deterioration of the nutritional status. Accordingly, the systematic screening for malnutrition and individual prevention and treatment has great potential to reduce the duration of hospitalisation, the probability of complications, the risk of rehospitalisation, as well as morbidity and mortality [25,26]. A valid predictor of poor nutritional status and associated mortality is the Nutritional Risk Score (NRS, range: 1-7). For each point increase in NRS, there was a significant stepwise increase in risk of 30-day mortality (adjusted Hazard Ratio (HR) of 1.22 and 180day mortality of HR 1.37)[26,27]. The EFFORT study systematically determined the effect of nutritional support in medical inpatients at nutritional risk based on the NRS. On this basis, individual nutritional goals were defined and malnutrition diagnosed at hospital admission was treated orally, enterally and parenterally according to a predefined algorithm. The focus was on meeting caloric and protein requirements, micronutrient supply and personalised nutritional goals. To reach nutritional goals, an individual nutritional plan was developed by a trained registered dietician including oral nutrition provided by the hospital combined with oral nutritional supplements. Depending on whether requirements could be met, a further increase in nutritional support to enteral tube feeding or parenteral feeding was introduced [25,28].
A subgroup analysis of the EFFORT trial found reduced cardiovascular morbidity and mortality when patients with heart failure received nutritional support. The NOURISH trial assessed nutritional status with the validated subjective global assessment (SGA) tool [29]. The study found no reduction in rehospitalisation in malnourished heart failure patients after oral nutritional supplement with beta-hydroxy-beta-methylbutyrate (HMBONS) and protein in addition to standard treatment, but the intervention reduced 90day mortality and improved postdischarge nutritional status. In addition, the number needed to treat (NNT) for HMB-ONS was low (NNT = 20.3) suggesting that provision of HMB-ONS to malnourished heart failure patients may be an effective strategy to improve current standard nutritional care [30]. The demographic change with an aging population stresses the need for early detection and prevention of malnutrition in vulnerable populations in the clinical but also in the home care setting. Nutritional approaches based on individual micro- and macronutrient status, glucose metabolism, body composition as well as digestion and absorption capacity – including microbiota properties – may help to further personalise dietary measures in order to increase treatment efficiency and improve patient outcomes. Furthermore, comprehensive evaluation of psychosocial, demographic and cultural preferences allows to establish sustainable rapport and trust between the patient and the provider [31,32].
Key points
Decreasing cardiovascular mortality should not hide the fact that heart failure is common in Switzerland. As the population ages demographically, the burden of disease will continue to rise.
Depending on the stage of heart failure, nutrition can influence the development and progression of the disease in different ways: from over-nutrition in early, to malnutrition in advanced stages.
An undersupply of energy, protein and individual micronutrients is common in hospitalised heart failure patients. It is associated with an increased risk of complications, rehospitalisation and mortality.
In hospitalised heart failure patients, screening for malnutrition and nutritional interventions in at-risk patients can significantly reduce complications and mortality.