Definition of clinical and subclinical atrial fibrillation
Atrial fibrillation (AF) is electrically defined as an uncoordinated, supraventricular tachyarrhythmia with irregular atrioventricular conduction (and hence irregular R-R intervals on the electrocardiogram) and no visible distinct P waves on a surface ECG [1]. Diagnosis of clinical AF requires documentation of such an arrhythmia on either a 12-lead ECG (independently of the episode duration) or on a singlelead ECG for at least 30 seconds, independently of symptoms related to AF according to the updated guidelines of the European Society of Cardiology [1]. So-called atrial high-rate episodes (AHREs) and subclinical AF have to be differentiated from clinical AF [1,2]. For both entities, it is of paramount importance that the patient is asymptomatic (in regard to the documented episodes) and that there is no previous documentation of clinical AF. AHREs are atrial tachyarrhythmias with a rate of >175–190 bpm detected by a cardiac implanted electronic device (CIED) with an atrial lead. Subclinical atrial fibrillation (SCAF) includes AHREs that are confirmed by inspection of the tracing to fulfillthe electrical definition of AF, or AF episodes detected by an insertable cardiac monitor or a wearable monitor that do not yet fulfillthe criteria for clinical AF (i.e., are less than 30 seconds) [1]. There is no uniform definition of duration of AHRE/SCAF; depending on the literature it ranges from 20 seconds to more than 24 hours [1–3].
Prevalence of subclinical atrial fibrillation
As for clinical atrial fibrillation, there is no absolute prevalence of SCAF because it depends on the population studied, the duration of screening and the type of screening tool. In the Apple Heart Study, more than 410,000 healthy subjects were enrolled. By using an Apple Watch®, an irregular pulse notification was observed in 0.52% of the entire population [6]. This number increases if the subject is older or has a higher CHA2DS2-VASc-score; the positive predictive value of such an irregular pulse notification to represent true AF was 84% [6]. If not a random, healthy population is being studied, but rather patients with a higher risk profile, the prevalence of SCAF increases. Indeed, the ASSERT-2 study found subclinical AF longer than 5 minutes (an implantable loop recorder was used for screening) in 39% of patients after an arterial thromboembolic event [7]. As mentioned, not only the risk of the population studied influences the prevalence of SCAF, but very importantly also the screening tool. The EMBRACE (using a 30-day external monitor) [8] and the CRYSTAL AF (using an implantable cardiac monitor) [9] trials both demonstrated that the rate of detection of atrial arrhythmias is significantly higher compared with a 24-hour Holter monitoring. And, last but not least, the duration definition for SCAF used also has a major impact on the likelihood of identifying SCAF: A meta-analysis of trials using either implantable or external cardiac monitors in patients after an ischaemic cerebral event demonstrated a rate of atrial arrhythmias (longer than 12 hours duration) of 11.5% [10], while the ASSERT-2 study using a minimum duration of 5 minutes for the definition of SCAF on implantable loop recorders found a much higher prevalence of SCAF [7].
Relation between SCAF and ischaemic stroke
A recent meta-analysis has demonstrated that SCAF is associated with an increased risk for stroke (2.4-fold increase, 95% confidence interval [CI] 1.8–3.3; p <0.001) [11]. However, definition of SCAF was not uniform between the studies included in this meta-analysis, which is relevant because further sub-analyses of some trials have confirmed that the risk of stroke is related to the duration of SCAF. In the ASSERT trial, it has been shown that the bulk of stroke events occurred in patients with more than 24 hours of SCAF [12], whereas episodes lasting less than 20 seconds were not associated with clinical events [13]. In between these two “extremes”, there is most likely a gradual increase in the risk of stroke, and the relevance of SCAF duration needs to be seen in the context of traditional risk factors associated with stroke embedded in the CHA2DS2- VAScscore. For example, in subjects with a low CHA2DS2-VASc-score of 1, the risk for stroke is significantly increased only if the duration of SCAF is ≥24 hours, whereas with a CHA2DS2VASc-score of ≥2, episodes of SCAF lasting only 5 minutes are associated with an increased risk for stroke [14].
Stroke prevention in patients with subclinical atrial fibrillation
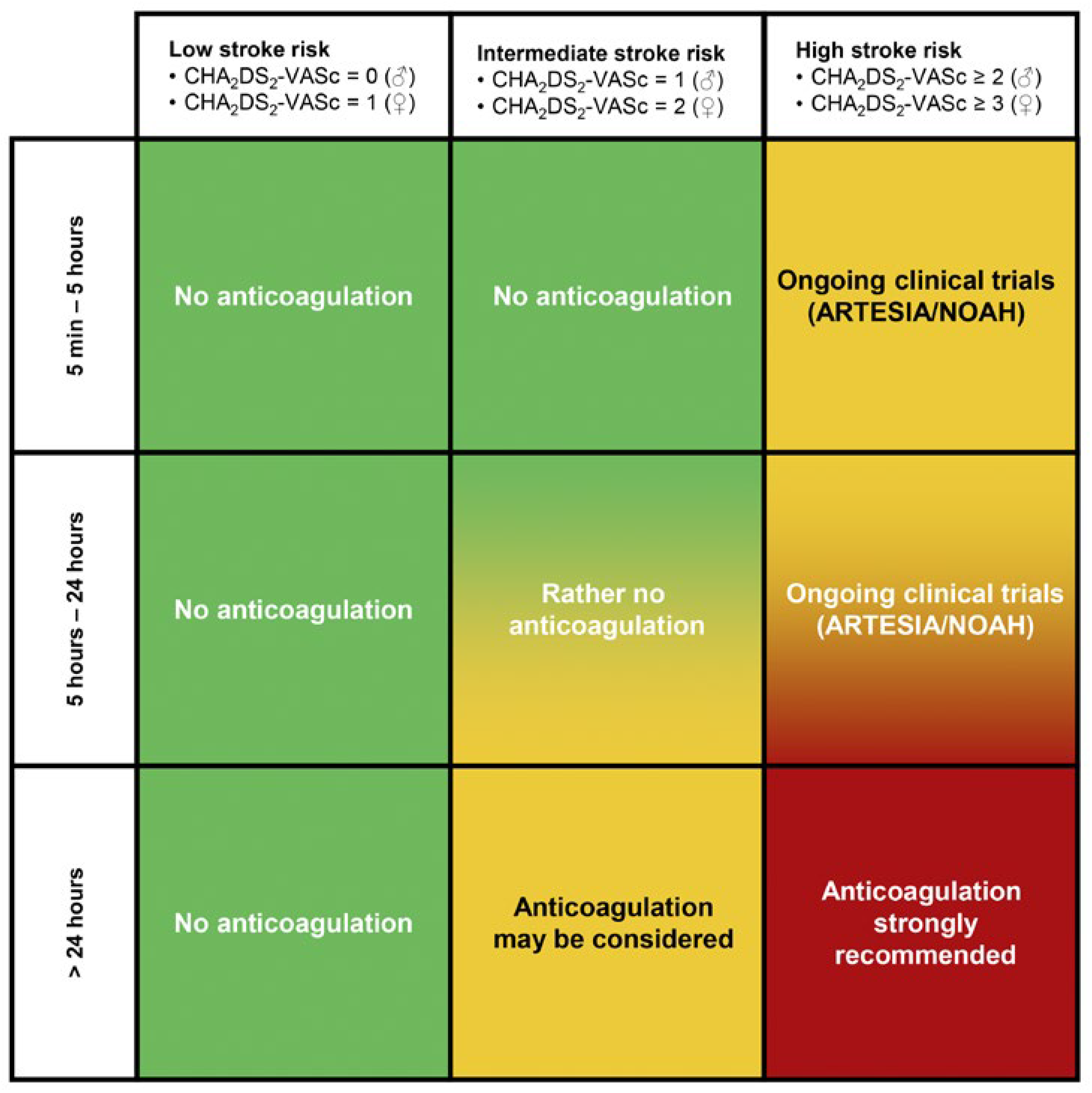
Unfortunately, the optimal approach to identifying the population of patients with SCAF who may benefit from anticoagulation to reduce the risk for stroke is still uncertain. As always, a balance has to be found between the risk for ischaemic thromboembolic events and the risk for bleeding complications. As long as definitive evidence is missing, duration of SCAF in combination with the risk for stroke calculated by the CHA2DS2- VASc-score is the most reasonable way to deal with this issue (
Figure 1). Patients with a SCAF duration of 24 hours or more together with an increased risk for stroke should undergo oral anticoagulation. On the other hand, for patients with short runs of SCAF (cut-off 5–6 minutes), anticoagulation seems not to significantly decrease the risk for stroke as demonstrated by the LOOP study [15]. For the patients in between, i.e., with SCAF episodes 5–24 hours, treatment should be chosen in a shared decision-making, balancing the patient’s goal and preference, and individual thromboembolic and bleeding risk. Ongoing clinical trials (ARTESIA [16] and NOAH [17]) will hopefully clarify in the near future the best treatment options in these patients.
Conclusion
Atrial fibrillation and subclinical AF are common findings due to improved detection tools as well as the higher prevalence of cardiovascular risk factors leading to these supraventricular arrhythmias. Although subclinical AF per definition is not causing thromboembolic events, it carries a potential risk for them and anticoagulation needs to be discussed with the patient. As long as data from randomised clinical trials are not yet available to select which patients will benefit from such a treatment, factors such as duration of subclinical AF as well as the risk for stroke (measured by CHA2DS2-VASc-Score) guide the decision to start anticoagulation or not.
Disclosure statement
AB has received consultant and / or speaker fees from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cook Medical, Daiichi Sankyo, Medtronic, Pfizer, and Spectranetics/ Philipps.
Recommended references
1 Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021 Feb 1;42(5):373-498. 10.1093/eurheartj/ehaa612.
7 Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJ, et al.; ASSERT-II Investigators. Subclinical Atrial Fibrillation in Older Patients. Circulation. 2017 Oct;136(14):1276–83. 10.1161/CIRCULATIO-NAHA.117.02884528778946
8 Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al.; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014 Jun;370(26):2467–77. 10.1056/NEJMoa131137624963566
13 Swiryn S, Orlov MV, Benditt DG, DiMarco JP, LloydJones DM, Karst E, et al.; RATE Registry Investigators. Clinical Implications of Brief Device-Detected Atrial Tachyarrhythmias in a Cardiac Rhythm Management Device Population: Results from the Registry of Atrial Tachycardia and Atrial Fibrillation Episodes. Circulation. 2016 Oct;134(16):1130–40. 10.1161/CIRCULATIO-NAHA.115.02025227754946
14 Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009 Mar;20(3):241–8. 10.1111/j.1540-8167.2008.01320.x19175849
© 2023 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.