Early Degeneration of the Sorin Mitroflow Aortic Bioprosthesis †
Abstract
Introduction
“The surgeon carries an important responsibility in protecting the patient from valve degeneration”Flameng W, et al. (2014)
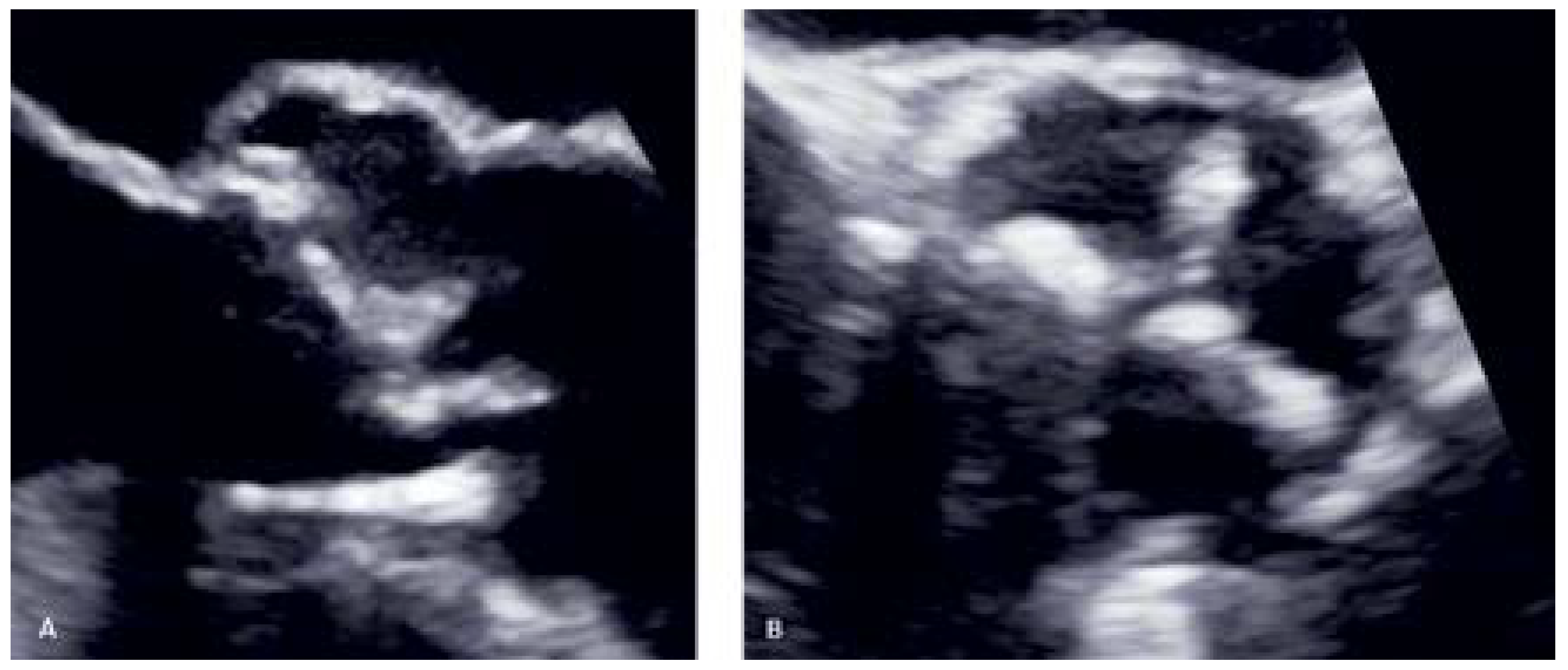
Case vignette (Patient no. 2)
Methods
Chart review
Echocardiographic review
Statistical analysis
Results
Clinical findings
Echocardiographic findings
Discussion
Limitations
Conclusion
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet. 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Gao, H.; Chambers, J.; Moat, N.; Murphy, G.; Pagano, D.; et al. Aortic valve surgery: Marked increases in volume and significant decreases in mechanical valve use—An analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National database. J Thorac Cardiovasc Surg. 2011, 142, 776–782.e3. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.J.; Shuhaiber, J.; Salemi, A.; Isom, O.W.; Sedrakyan, A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J Thorac Cardiovasc Surg. 2015, 149, 1262–1269.e3. [Google Scholar] [CrossRef] [PubMed]
- Fujita, B.; Ensminger, S.; Bauer, T.; Möllmann, H.; Beckmann, A.; Bekeredjian, R.; GARY Executive Board; et al. Trends in practice and outcomes from 2011 to 2015 for surgical aortic valve replacement: An update from the German Aortic Valve Registry on 42 776 patients. Eur J Cardiothorac Surg. 2018, 53, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Fatima, B.; Mohananey, D.; Khan, F.W.; Jobanputra, Y.; Tummala, R.; Banerjee, K.; et al. Durability data for bioprosthetic surgical aortic valve. A systematic review. JAMA Cardiol. 2019, 4, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, W.R.; Yankah, C.A.; Lorusso, R.; Benhameid, O.; Forgie, R.; Ling, H.; et al. Clinical and hemodynamic performance of the Sorin Mitroflow pericardial bioprosthesis. 9 December 2011. Available online: www.intechopen.com. [CrossRef]
- Balsam, L.B.; De Anda, A., Jr. The Mitroflow aortic valve: A past, present, and future illuminated. J Thorac Cardiovasc Surg. 2017, 153, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Pomar, J.L.; Jamieson, W.R.; Pelletier, L.C.; Gerein, A.N.; Castellá, M.; Brownlee, R.T. Mitroflow pericardial bioprosthesis: Clinical performance to ten years. Ann Thorac Surg. 1995, 60 (Suppl. 2), S305–S309. [Google Scholar] [CrossRef] [PubMed]
- Loisance, D.; Zouari, M.; Leandri, J.; Hillion, M.L.; Cachera, J.P. The Mitroflow pericardial valve. First five years of follow-up evaluation. ASAIO Trans. 1989, 35, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Crown PRT Aortic Pericardial Heart Valve with PR Treatment. Minor Design Modifications to Mitroflow DL. Jan 2016. FDA Premarket Approval (PMA), FDA Home, Medical Devices, Database. URL.
- Rodriguez-Gabella, T.; Voisine, P.; Puri, R.; Pibarot, P.; Rodés-Cabau, J. Aortic bioprosthetic valve durability: Incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration. J Am Coll Cardiol. 2017, 70, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Marro, M.; Kossar, A.P.; Xue, Y.; Frasca, A.; Levy, R.J.; Ferrari, G. Noncalcific mechanisms of bioprosthetic structural valve degeneration. J Am Heart Assoc. 2020, 10, e018921. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Dumesnil, J.G. Prosthetic heart valves: Selection of the optimal prosthesis and long-term management. Circulation. 2009, 119, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- De Paulis, R.; D’Aleo, S.; Bellisario, A.; Salica, A.; Weltert, L.P.; Scaffa, R.; et al. The fate of small-size pericardial heart valve prostheses in an older patient population. J Thorac Cardiovasc Surg. 2017, 153, 31–39.e2. [Google Scholar] [CrossRef] [PubMed]
- Anantha Narayanan, M.; Suri, R.M.; Ugur, M.; Greason, K.L.; Stulak, J.M.; Dearani, J.A.; et al. Predictors of survival and modes of failure after Mitroflow aortic valve replacement in 1,003 adults. Ann Thorac Surg. 2015, 100, 560–567. [Google Scholar] [CrossRef] [PubMed]
- ISTHMUS Investigators. The Italian study on the Mitroflow postoperative results (ISTHMUS): A 20-year, multicentre evaluation of Mitroflow pericardial bioprosthesis. Eur J Cardiothorac Surg. 2011, 39, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Thulin, L.I.; Thilén, U.J.; Kymle, K.A.; Thulin, L.I.; Ulf, J.; Thilén, K.A. Mitroflow pericardial bioprosthesis in the aortic position. Low incidence of structural valve deterioration in elderly patients during an 11-year follow-up. Scand Cardiovasc J. 2000, 34, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Zittermann, A.; Schulte-Eistrup, S.; Koertke, H.; Körfer, R. Mitroflow synergy prostheses for aortic valve replacement: 19 years experience with 1,516 patients. Ann Thorac Surg. 2005, 80, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, J.; Gudbjartsson, T.; Thulin, L.I. Long-term outcome of the MitroFlow pericardial bioprosthesis in the elderly after aortic valve replacement. J Heart Valve Dis. 2006, 15, 197–202. [Google Scholar] [PubMed]
- Mosquera, V.X.; Bouzas-Mosquera, A.; Velasco-García, C.; Muñiz, J.; Estévez-Cid, F.; Portela-Torron, F.; et al. Long-term outcomes and durability of the Mitroflow aortic bioprosthesis. J Card Surg. 2016, 31, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Benhameid, O.; Jamieson, W.R.; Castella, M.; Carrier, M.; Pomar, J.L.; Germann, E.; et al. CarboMedics Mitroflow pericardial aortic bioprosthesis—performance in patients aged 60 years and older after 15 years. Thorac Cardiovasc Surg. 2008, 56, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Yankah, C.A.; Pasic, M.; Musci, M.; Stein, J.; Detschades, C.; Siniawski, H.; et al. Aortic valve replacement with the Mitroflow pericardial bioprosthesis: Durability results up to 21 years. J Thorac Cardiovasc Surg. 2008, 136, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Yankah, C.A. Mitroflow pericardial aortic bioprosthesis in patients younger than 60 years. J Thorac Cardiovasc Surg. 2010, 140, e83–e84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alvarez, J.R.; Sierra, J.; Vega, M.; Adrio, B.; Martinez-Comendador, J.; Gude, F.; et al. Early calcification of the aortic Mitroflow pericardial bioprosthesis in the elderly. Interact Cardiovasc Thorac Surg. 2009, 9, 842–846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saleeb, S.F.; Newburger, J.W.; Geva, T.; Baird, C.W.; Gauvreau, K.; Padera, R.F.; et al. Accelerated degeneration of a bovine pericardial bioprosthetic aortic valve in children and young adults. Circulation. 2014, 130, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sénage, T.; Le Tourneau, T.; Foucher, Y.; Pattier, S.; Cueff, C.; Michel, M.; et al. Early structural valve deterioration of Mitroflow aortic bioprosthesis: Mode, incidence, and impact on outcome in a large cohort of patients. Circulation. 2014, 130, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Butany, J.; Feng, T.; Luk, A.; Law, K.; Suri, R.; Nair, V. Modes of failure in explanted mitroflow pericardial valves. Ann Thorac Surg. 2011, 92, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.H.; Hjortdal, V.; Modrau, I.S.; Jensen, H.; Kimose, H.H.; Terp, K.; et al. Durability after aortic valve replacement with the Mitroflow versus the Perimount pericardial bioprosthesis: A single-centre experience in 2393 patients. Eur J Cardiothorac Surg. 2016, 49, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Aasbjerg, K.; Mortensen, P.E.; Nørgaard, M.A.; Rytgaard, H.C.; Gerds, T.A.; Søgaard, P.; et al. Comparison of survival after aortic valve replacement with Mitroflow or Perimount Prostheses. Semin Thorac Cardiovasc Surg. 2019, 31, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Theologou, T.; Harky, A.; Shaw, M.; Harrington, D.; Kuduvalli, M.; Oo, A.; et al. Mitroflow and Perimount Magna 10 years outcomes a direct propensity match analysis to assess reintervention rates and long follow-up mortality. J Card Surg. 2019, 34, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gabella, T.; Voisine, P.; Dagenais, F.; Mohammadi, S.; Perron, J.; Dumont, E.; et al. Long-term outcomes following surgical aortic bioprosthesis implantation. J Am Coll Cardiol. 2018, 71, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Axtell, A.L.; Chang, D.C.; Melnitchouk, S.; Jassar, A.S.; Tolis, G.; Villavicencio, M.A.; et al. Early structural valve deterioration and reoperation associated with the mitroflow aortic valve. J Card Surg. 2018, 33, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Hickey, G.L.; Bridgewater, B.; Grant, S.W.; Deanfield, J.; Parkinson, J.; Bryan, A.J.; et al. National Registry data and record linkage to inform postmarket surveillance of prosthetic aortic valve models over 15 years. JAMA Intern Med. 2017, 177, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Furnary, A.P.; Li, H.F.; Grunkemeier, G.L. Bioprosthetic aortic valve durability: A meta-regression of published studies. Ann Thorac Surg. 2017, 104, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.J.; Bühlmann Lerjen, E.; Pellegrini, D.; Eberli, F.R. Sudden cardiac arrest during emergency caesarean delivery in a 31-year-old woman, due to accelerated structural valve degeneration of an aortic valve bioprosthesis. BMJ Case Rep. 2015, 2015, bcr2015212575. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.; Hernández-Vaquero, D.; Silva, J.; Pascual, I.; de la Hera, J.M.; León, V.; et al. Real structural valve deterioriation of the Mitroflow aortic prosthesis: Competing risk analysis. Rev Esp Cardiol (Engl Ed). 2017, 70, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Sénage, T.; Gillaizeau, F.; Le Tourneau, T.; Marie, B.; Roussel, J.C.; Foucher, Y. Structural valve deterioration of bioprosthetic aortic valves: An underestimated complication. J Thorac Cardiovasc Surg. 2019, 157, 1383–1390.e5. [Google Scholar] [CrossRef] [PubMed]
- Issa, I.F.; Poulsen, S.H.; Waziri, F.; Torp Pedersen, C.; Nielsen, P.H.; Riber, L.; et al. Structural valve deterioration in the Mitroflow biological heart valve prosthesis. Eur J Cardiothorac Surg. 2018, 53, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Leon, M.B.; et al. On behalf of VIVID Investigators. Standardized definition of structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. White Paper. Circulation 2018, 137, 388–399. [Google Scholar]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2017, 52, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Flameng, W.; Rega, F.; Vercalsteren, M.; Herijgers, P.; Meuris, B. Antimineralization treatment and patient-prosthesis mismatch are major determinants of the onset and incidence of structural valve degeneration in bioprosthetic heart valves. J Thorac Cardiovasc Surg. 2014, 147, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Schulz, J.; Roumieh, M.; Fleissner, F.; Ismail, I.; Tudorache, I.; et al. Long-term results of the Mitroflow aortic pericardial bioprosthesis in over 800 patients: Limited durability and mechanisms of dysfunction. Eur J Cardiothorac Surg. 2017, 52, 264–271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruvolo, G.; Pisano, C.; Balistreri, C.R.; Maresi, E.; Triolo, O.F.; Argano, V.; et al. Early structural degeneration of Mitroflow aortic valve: Another issue in addition to the mismatch? J Thorac Dis. 2018, 10, E270–4. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Lucas, A.; Permanyer, E.; Pérez, M.L.; Gracia-Baena, J.M.; Ríos, R.; Casós, K.; et al. Effect of bioprostheses anti-calcification treatment: Comparative follow-up between Mitroflow LX and Magna pericardial xenografts using a propensity score-weighted analysis. Interact Cardiovasc Thorac Surg. 2017, 24, 335–341.pmid. [Google Scholar] [CrossRef] [PubMed]
- Bassano, C.; Gislao, V.; Bovio, E.; Melino, S.; Tropea, I.; Saitto, G.; et al. An unexpected risk factor for early structural deterioration of biological aortic valve prostheses. Ann Thorac Surg. 2018, 105, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Koene, B.; Timmermans, N.; Soliman-Hamad, M.; van Straten, A. Reintervention after aortic valve replacement: Comparison of 3 aortic bioprostheses. Ann Thorac Surg. 2020, 110, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Biancari, F.; Valtola, A.; Juvonen, T.; Husso, A.; Dahlbacka, S.; Laakso, T.; et al. Trifecta versus Perimount Magna Ease aortic valve prostheses. Ann Thorac Surg. 2020, 110, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Yongue, C.; Lopez, D.C.; Soltesz, E.G.; Roselli, E.E.; Bakaeen, F.G.; Gillinov, A.M.; et al. Durability and performance of 2298 Trifecta aortic valve prostheses: A propensity-matched analysis. Ann Thorac Surg. 2021, 111, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.; Alalawi, Z.; Voss, S.; Boehm, J.; Krane, M.; Vitanova, K. Different rates of bioprosthetic aortic valve failure with Perimount and Trifecta Bioprostheses. Front Cardiovasc Med. 2022, 8, 822893. [Google Scholar] [CrossRef] [PubMed]
- Gurvitch, R.; Cheung, A.; Bedogni, F.; Webb, J.G. Coronary obstruction following transcatheter aortic valve-in-valve implantation for failed surgical bioprostheses. Catheter Cardiovasc Interv. 2011, 77, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, V.X.; González-Barbeito, M.; Bouzas-Mosquera, A.; Herrera-Noreña, J.M.; Velasco, C.; Salgado-Fernández, J.; et al. Efficacy and safety of transcatheter valve-in-valve replacement for Mitroflow bioprosthetic valve dysfunction. J Card Surg. 2018, 33, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.W.; Ye, J.; Dvir, D.; Wood, D.A.; Webb, J.G.; Dvir, D.D.; Wood, D.A.; Webb, J.G. Aortic valve-in-valve in externally mounted bioprosthesis. A safe treatment option for bioprosthetic structural valve dysfunction. Innovations (Phila). 2018, 13, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Mercanti, F.; Rosseel, L.; Neylon, A.; Bagur, R.; Sinning, J.M.; Nickenig, G.; et al. Chimney stenting for coronary occlusion during TAVR. Insights from the Chimney registry. JACC Cardiovasc Interv. 2020, 13, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Kudsk, J.E.; Andersen, A.; Therkelsen, C.J.; Christensen, E.H.; Jensen, K.T.; Krusell, L.R.; et al. High-pressure balloon fracturing of small dysfunctional Mitroflow bioprostheses facilitates transcatheter aortic valve-in-valve implantation. EuroIntervention. 2017, 13, e1020–5. [Google Scholar] [CrossRef] [PubMed]
- Carabello, B.A. Bioprosthetic valve leaflet thrombosis. How the past may inform us about the future. J Am Coll Cardiol. 2020, 75, 867–869. [Google Scholar] [CrossRef] [PubMed]

| Baseline characteristics | Cohort (n = 54) |
|---|---|
| Non-significant SVD, n | 23 |
| Male sex, n (%) | 16 (70) |
| Average age, years (range) | 76.5 (52–87) |
| Significant SVD, n | 31 |
| Male sex, n (%) | 14 (45) |
| Sorin Mitroflow LXA prosthesis (%) | 18 (58) |
| Sorin Mitroflow DLA prosthesis (%) | 13 (42) |
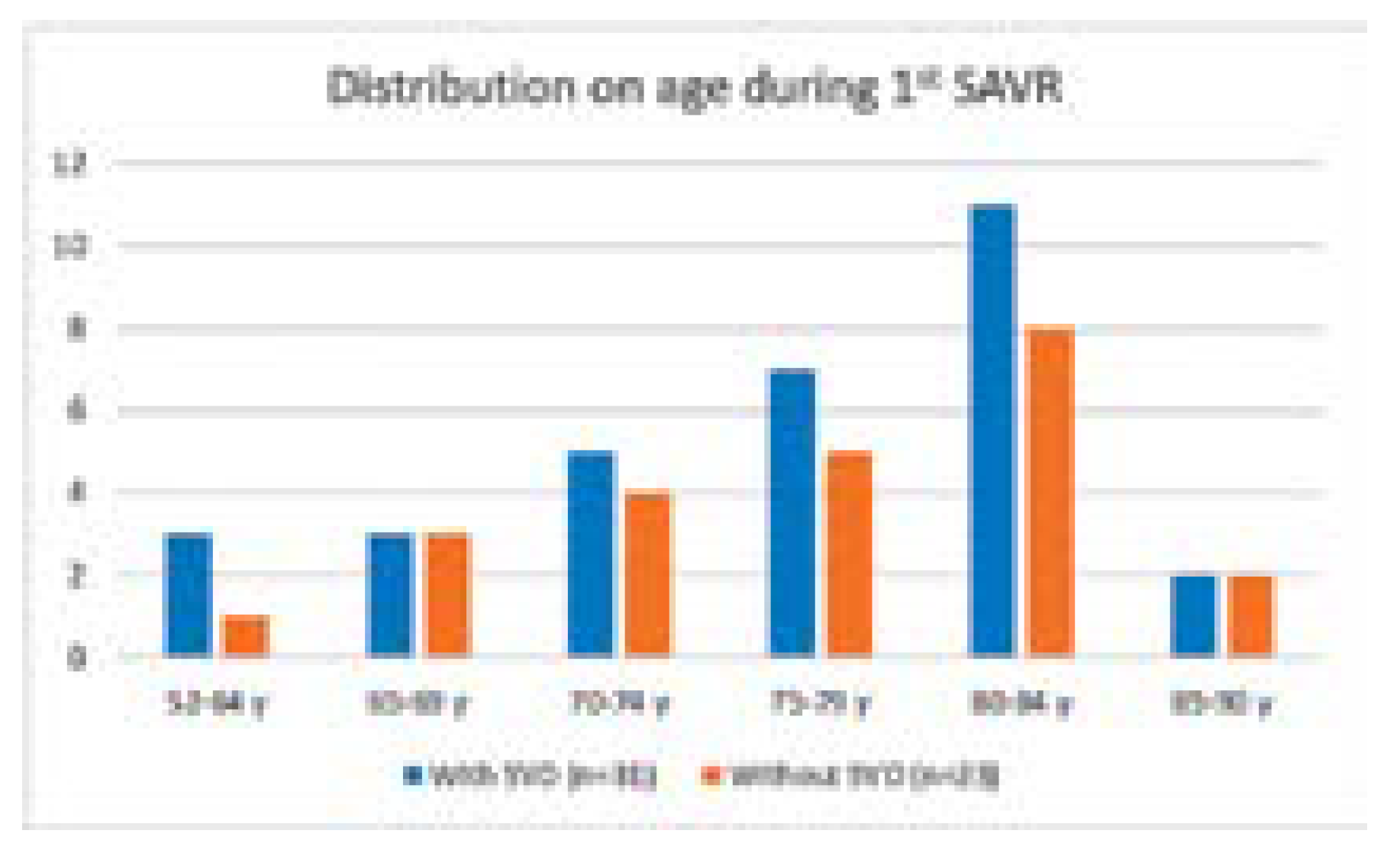
| Age at SAVR, years (range) | 76.9 (63–89) |
| Age at diagnosis of SVD, years (range) | 81.9 (69–92) |
| Surgical procedure | n (%) |
| Isolated AVR (maze procedure 2x) | 18 (58) |
| AVR and CABG | 7 (23) |
| AVR and aortoplasty | 5 (16) |
| AVR and MVR | 1 (3) |
| Clinical signs of SVD | |
| Murmur | 18 (58) |
| Shortness of breath | 3 (10) |
| Heart failure | 20 (65) |
| Heart failure hospitalisation | 15 (48) |
| Patient age and time intervals in years | Average (range) |
|---|---|
| Time interval in years from index operation to | |
| Diagnosis of moderate/severe SVD (n = 31) | 5.3 (2.4–9.2) |
| End of follow-up without intervention (n = 4) | 8.7 (6.8–9.6) |
| Reintervention (n = 16) | 6.9 (5.2–11.9) |
| Death (n = 11) | 7.0 (5.2–9.9) |
| Patients without re-intervention (n = 15) | 88.5 (84–95) |
| Patients alive (n = 4) | 87.0 (84–90) |
| Patients deceased (n = 11) | 89.0 (84–95) |
| Patients with re-intervention (n = 16) | 79.5 (69–91) |
| SAVR (n = 8) | 77.9 (69–84) |
| TAVI (n = 7) | 79.7 (76–86) |
| Transapical AVR (n = 1) | 91 |
| Pat. No. | Surgery | Mitroflow, mm, model (LXA or DLA) | Degree of SVD | AV max (m/s) | AVA Index | DVI | Death after first sign of SVD (months) | Implication, re-operation procedure |
|---|---|---|---|---|---|---|---|---|
| 1 | AKE, ACBOPx5 | 25, LXA | Severe | 2.74 | 1.00 | 0.52 | TAVI, valve in valve | |
| 2 | AKE, ACBOPx1 | 23, LXA | Severe | 5.00 | 0.24 | 0.20 | Redo SAVR Perimount Magna 21 mm | |
| 3 | AKE | 21, LXA | Moderate | 3.00 | 0.58 | 0.32 | 24 | No intervention, death |
| 4 | AKE | 23, LXA | Moderate | 2.40 | 0.73 | 0 | No intervention, death | |
| 5 | AKE, ACBOPx4 | 23, LXA | Moderate | 3.00 | 0.68 | 0.32 | 65 | No intervention, death |
| 6 | AKE | 23, LXA | Severe | 3.50 | 0.46 | 0.40 | Redo SAVR Perimount Magna 21 mm | |
| 7 | AKE, ACBOPx2 | 23, DLA | Severe | 4.00 | 0.62 | 0.22 | 0 | No intervention, death |
| 8 | AKE | 23, LXA | Moderate | 4.20 | 0.53 | 0.24 | Watchful waiting, 84 years old | |
| 9 | AKE | 27, LXA | Severe | 0.56 | Redo SAVR Perimount Magna 23 mm | |||
| 10 | AKE, ACBOPx1 | 25, DLA | Severe | 4.22 | 0.30 | 0.20 | Redo SAVR Perimount Magna 23 mm | |
| 11 | AKE, MKR | 29, DLA | Severe | 5.17 | 0.25 | 0.10 | TAVI, valve in valve | |
| 12 | AKE, MAZE | 27, DLA | Moderate | 3.05 | 1.13 | 0.43 | 0 | No intervention, death |
| 13 | AKE | 23, DLA | Severe | 3.80 | 0.61 | 0.26 | Redo SAVR, Perimount Magna 21 mm | |
| 14 | AKE | 27, DLA | Severe | 0.26 | TAVI, valve in valve | |||
| 15 | AKE, MAZE | 27, LXA | Severe | 2.80 | 1.14 | 0.64 | Redo SAVR, Sorin Carbomedics 25 mm | |
| 16 | AKE | 25, LXA | Severe | 4.40 | 0.45 | 0.37 | TAVI, valve in valve | |
| 17 | AKE | 23, DLA | Severe | 3.60 | 0.60 | 27 | No intervention, death | |
| 18 | AKE, ACBOPx2 | 23, LXA | Severe | 4.24 | 0.56 | 0.30 | 12 | No intervention, death |
| 19 | AKE | 23, LXA | Moderate | 3.60 | 0.71 | 0.34 | 0 | No intervention, death |
| 20 | AKE | 23, LXA | Moderate | 2.80 | 0.73 | 0.35 | Wacthful waiting, 87 years old | |
| 21 | AKE, Rohrproth. | 25, DLA | Severe | 2.78 | 1.41 | 0.63 | Redo SAVR, Perimount Magna 23 mm | |
| 22 | AKE, AoReduk. | 25, LXA | Severe | 3.16 | 0.54 | 0.24 | Watchful waiting, 86 years old | |
| 23 | AKE | 21, DLA | Severe | 5.40 | 0.28 | 0.18 | TAVI, valve in valve | |
| 24 | AKE | 27, LXA | Severe | 2.80 | TAVI, valve in valve | |||
| 25 | AKE | 23, DLA | Severe | 3.61 | 0.32 | 48 | No intervention, death | |
| 26 | AKE, Ao Reduk. | 25, LXA | Severe | 4.04 | 0.47 | 0.21 | Redo SAVR, Sorin Crown PRT | |
| 27 | AKE | 23, DLA | Severe | 3.45 | 0.59 | 0.24 | Watchful waiting, 90 years old | |
| 28 | AKE, Ao Reduk. | 25, LXA | Moderate | 3.00 | 0.51 | 0.34 | 40 | No intervention, death |
| 29 | AKE, ACBOPx4 | 25, DLA | Severe | 2.80 | 0.56 | TAVI, valve in valve | ||
| 30 | AKE | 21, LXA | Moderate | 2.73 | 0.59 | 0.36 | 27 | No intervention, death |
| 31 | AKE, suprac. Ersatz | 23, DLA | Severe | 3.00 | 0.80 | 0.37 | Transapical AVR, valve in valve |
| Young age |
| Severe reduced kidney function with haemodialysis |
| Patient prosthesis mismatch |
| Infective endocarditis |
| Early valve thrombosis (?) |
| ∆p mean >50% of baseline |
| Thickened cusps with or without calcification |
| Reduced mobility of one or more cusps |
© 2022 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Widmer, F.; Schliephake, F.; Mattle, D.; Federmann, M. Early Degeneration of the Sorin Mitroflow Aortic Bioprosthesis. Cardiovasc. Med. 2022, 25, 116. https://doi.org/10.4414/cvm.2022.02211
Widmer F, Schliephake F, Mattle D, Federmann M. Early Degeneration of the Sorin Mitroflow Aortic Bioprosthesis. Cardiovascular Medicine. 2022; 25(4):116. https://doi.org/10.4414/cvm.2022.02211
Chicago/Turabian StyleWidmer, Fritz, Florian Schliephake, Daniel Mattle, and Martin Federmann. 2022. "Early Degeneration of the Sorin Mitroflow Aortic Bioprosthesis" Cardiovascular Medicine 25, no. 4: 116. https://doi.org/10.4414/cvm.2022.02211
APA StyleWidmer, F., Schliephake, F., Mattle, D., & Federmann, M. (2022). Early Degeneration of the Sorin Mitroflow Aortic Bioprosthesis. Cardiovascular Medicine, 25(4), 116. https://doi.org/10.4414/cvm.2022.02211




