Abstract
INTRODUCTION: Current European guidelines recommend a multidisciplinary team approach in infective endocarditis in order to ensure adequate treatment and follow-up. The aim of this contribution is to describe the organisation of the Endocarditis Board at the University Hospital Zurich and report the initial outcome data for patients with short-term follow-up information. METHODS: The Endocarditis Board includes specialists in infectious diseases, cardiovascular surgery, cardiology, anaesthesiology, microbiology, nuclear medicine and cardiac imaging, neurology and pathology. Interactive weekly participation from all specialties guarantees integrated patient care. Consensus decisions are documented in the institutional electronic medical record. Systematic observational data collection is an important aspect of quality assurance and feedback. RESULTS: The Endocarditis Board became operational in May 2016. All cases of proven or suspected infective endocarditis primarily admitted or referred were discussed. Between May 2016 and December 2020, 595 consecutive patients were discussed, leading to 1145 discussion episodes. Based on detailed analysis, the diagnosis of infective endocarditis was rejected by consensus in 128 patients. Of the 467 patients with cardiovascular infections, 113 (24%) were female, median age was 68 years, 346 (70%) had an infective endocarditis (218 native valve infective endocarditis / 122 prosthetic valve infective endocarditis / 6 marantic), 73 (16%) had device-associated and 48 (10%) vascular graft infections. Gram-positive bacteria were predominant (Staphylococcus aureus (141, 30%); Coagulase-negative Staphylococcus (53, 11%); Streptococcus spp (120, 26%) and Enterococcus spp (51, 11%)). Surgery was performed in 190 (41%) patients. Thirty-day and 1-year mortality were 9.6% and 14%, respectively. CONCLUSION: Currently the Endocarditis Team and Endocarditis Board play a key role in the treatment of cardiovascular infections according to the European Guidelines and belong to the standard of care at our institution.
Introduction
Cardiovascular infections, and infective endocarditis in particular, are complex diseases. Even if treated properly, current studies still show high mortality in the acute phase (20–30%) [1,2,3,4]. In developing countries, infective endocarditis has a subacute and more chronic course, mainly due to underlying rheumatic heart disease. Treatment of streptococcal throat infections as well as endocarditis prophylaxis have almost eradicated this disease among the native population in western countries. However, occasional rheumatic heart disease cases are diagnosed among people with a migration background [5].
Epidemiological studies have shown changes in patient characteristics and pathogens over past decades with a current incidence of up to 10 cases per 100,000 patients/year [6,7,8]. Native valve infective endocarditis (NVIE) is still the most frequent form [9]. However, the incidence of prosthetic valve infective endocarditis (PVIE), cardiac device- and vascular graft-related endocarditis has increased in recent years, mainly due to more frequent surgical and interventional procedures in patients having received a prior cardiovascular implant and broader indications [3,9]. Also, the increase of transcatheter interventions, implantation of repair devices and the rising number of patients with congenital heart disease reaching adulthood contribute to the risk of infection [10]. Diagnosis of infective endocarditis relies on the modified Duke criteria [11,12,13] (Table 1). Despite adequate antimicrobial treatment and diagnosis, cardiac surgery is required in 40–50% of cases [13]. A multidisciplinary team approach is currently recommended in tertiary care institutions [1,14,15]. Recent experiences suggest and support that a multidisciplinary team reduces mortality rates [1,2,14,15]. This approach was incorporated into the 2015 ESC Guidelines for the Management of Infective Endocarditis as a Class IIa B recommendation [12]. In this article, we aim to describe the work of the Endocarditis Board at the University Hospital of Zurich and to report the initial management and outcome data of patients with short-term follow-up information.

Table 1.
Modified Duke Criteria with task force recommendations [12].
Methods
Endocarditis Board mission and vision statement
In recent years, the value of an endocarditis board in the diagnosis and treatment has been well highlighted by individual institutions and scientific societies (such as the European Society of Cardiology [ESC]). Therefore, the Endocarditis Board was created in order to standardise and improve the quality of care for patients with infective endocarditis by developing a workflow algorithm and a multidisciplinary team to facilitate interdisciplinary communication, decrease the possibility of treatment errors, expedite surgical indications and to pursue continued quality improvement.
Setting
The University Hospital of Zurich is a tertiary care teaching hospital with about 900 beds and a large referral centre for heart surgery covering the northeastern part of Switzerland. The Endocarditis Board was established in 2016 and since then meets on a weekly basis. The meeting is scheduled as a hybrid event with either physical or virtual presence. Referring hospitals can participate by Skype™-based video conferencing services. The list of patients who are scheduled for discussion is e-mailed to the participants one day ahead of the next meeting. The list includes basic information such as demographics, responsible physician, valve affected, isolated pathogen (if any), antimicrobial therapy and available images. The core team is available at any time during the week for emergency and urgent cases.
Structure of the Endocarditis Board
The Endocarditis Board includes specialists in:
- Anesthesiology
- Cardiovascular Surgery
- Cardiology
- Infectious Disease
- Intensive Care
- Neurology
- Nuclear Medicine
- Pathology
The complexity of the disease may require the participation of other departments that are not listed above but are consulted when necessary. Research nurses play an important role and are actively involved in data abstraction, Endocarditis Board discussion compilation, blood sampling and patient support.
Workflow
Each case is presented and the likelihood of infective endocarditis is evaluated according to the modified Duke criteria.
Imaging findings such as transthoracic or transoesophageal echocardiography (TTE/TOE), computed tomography (CT) or positron emission tomography computed tomography (PET/CT) scans are presented by the corresponding specialists. Valve dysfunction is quantified, vegetation, fistula, abscesses and other pathological echocardiographic findings are identified, and cardiac function is documented. Patients without clear signs and a low probability of infective endocarditis are excluded, but always with the possibility of re-evaluation. After infective endocarditis is confirmed an appropriate treatment strategy is recommended, antimicrobial therapy and treatment duration are determined in accordance with international treatment guidelines. Additionally, the ideal timing of surgery, if required, is discussed. Once consensus on the treatment strategy is reached, the team issues an official statement, which is documented in the electronical medical record. If feasible, each case will be reassessed at the next Endocarditis Board and the outcome presented (Figure 1).
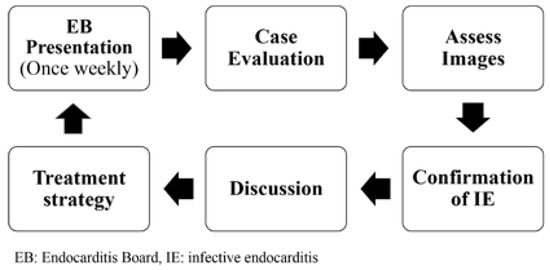
Figure 1.
Workflow.
Another goal is to support the continuity of outpatient management after hospital discharge and to gather follow-up information. After initial intravenous antimicrobial therapy according to microbiology results, patients may be discharged and allocated to outpatient parenteral antibiotic therapy [16] or referred to other centres for treatment continuation. This may eventually change in accordance with a recent trial, which supports oral continuation therapy for 4 weeks in selected groups of patients [17]. Most of the patients discussed at the Endocarditis Board meetings are also enrolled in our internal prospective infective endocarditis registry (ENVALVE).
When to refer a patient to a tertiary centre
Patients are frequently referred from other centres without cardiovascular surgery. Hence, it is important that referring clinics stay in continuous contact with the Endocarditis Board (Figure 2), in order to ascertain op-timal timing for transfer. Indications for transferring a patient to a tertiary referral centre are summarised in Table 2. For patients not meeting these criteria, periodic communication with the Endocarditis Board is recommended.
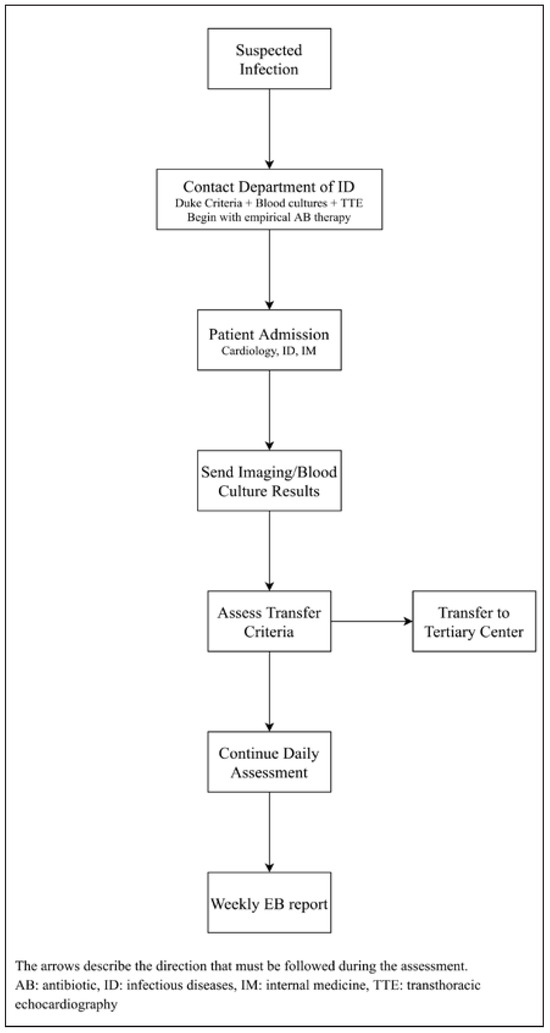
Figure 2.
Suggested management by referring clinics.

Table 2.
Indications for transfer to a tertiary centre.
Case management
Figure 3 depicts the suggested algorithm when infective endocarditis is suspected. The first recommendation in the case of clinically suspected infective endocarditis is to call the emergency medical service of the Department of Infectious Diseases and Hospital Epidemiology, as well as the Heart Team. Initially, three sets of blood cultures must be drawn. Empirical antimicrobial treatment starts as soon as blood cultures have been taken. Simultaneously or within 48 hours, a TTE should be performed in order to disclose typical signs of infective endocarditis and assess cardiac function. Patients with heart failure and/or cardiogenic shock, as well as those with PVIE and device-related infective endocarditis need to be admitted and daily assessment is mandatory in order to detect a worsening in the patient’s condition and be able to adjust treatment without any delay. TOE is performed in inconclusive cases when TTE cannot provide definite information, in PVIE and in those patients with an infected intracardiac device. An intraoperative TOE is also always performed. CT or MRI scans are performed to rule out cerebroand/or renovisceral embolism, but they not replace TTE/TOE examinations. Generally we perform whole body CT scans in all types of Staphylococcus aureus endocarditis to exclude peripheral embolisms and in mechanical aortic valve prosthesis endocarditis to exclude locoregional complications, which can be difficult to diagnose because of acoustic shadowing. PET/CT scans are mainly reserved for patients with prosthetic materials (PVIE, vascular graft- and device-associated infections) when endocarditis can not be confirmed by all other modalities and clinical suspicion remains high. Lately we tend to replace CT scans with PET/ CT scans in complex cases with multiple prosthetic materials (intracardiac and extracardiac), allowing locoregional complications/infections as well as peripheral embolisms and infections to be excluded.
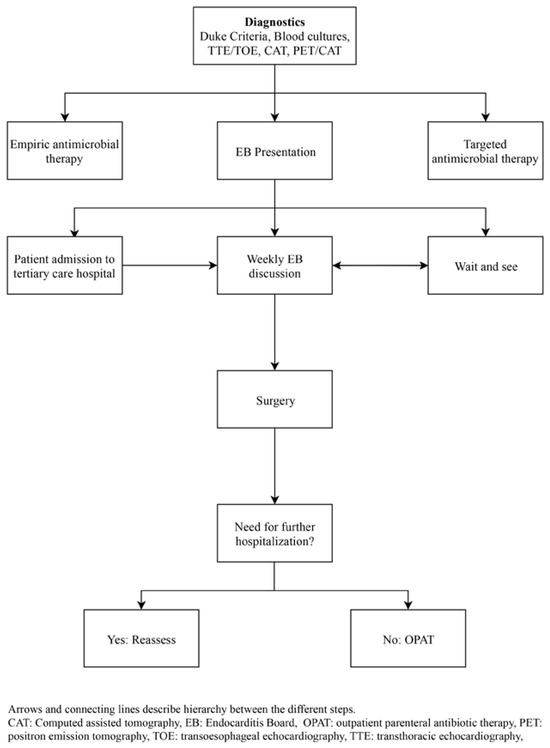
Figure 3.
Daily assessment (suspected infection).
Indication for and timing of surgery
Between 40% and 50% of all infective endocarditis-patients will need surgical treatment in the acute phase [8,13]. Three different scenarios are considered: emergency (within 24 hours), urgent (within a few days, <7 days) and elective (between 1 and 2 weeks) surgery. Indications for surgical treatment of infective endocarditis at any valve location are summarised in Table 3.

Table 3.
Indications and timing for surgery (ESC / Eurupean Association for Cardio-Thoracic surgery Guidelines [12]).
Ethics
The Zurich Cantonal Ethics Commission waived the necessity for a formal ethical evaluation based on the Swiss Federal Human Research Act. Due to the ethics waiver, no informed consent had to be requested. Moreover, a large number of the discussed patients are enrolled in our institutional prospective infective endocarditis registry ENVALVE, where all participants provide written informed consent.
Results
Time frame
The Endocarditis Board at the University Hospital Zurich started its activities in May 2016.
Patient assessment
Between May 2016 and December 2020, 595 consecutive patients with suspected infective endocarditis were discussed, leading to 1145 case-discussion episodes. The Endocarditis Board rejected by consensus the diagnosis of a cardiovascular infection in 128 patients. Of the 467 patients with cardiovascular infections (113 female, median age 68 years) 346 (70%) had an infective endocarditis (218 NVIE / 122 PVIE / 6 marantic), 73 (16%) had device-associated and 48 (10%) vascular graft infections. Concerning microbiology, Grampositive bacteria predominated (S. aureus (141, 30%); coagulase-negative staphylococci (53, 11%); Streptococcus spp (120, 26%) and Enterococcus spp (51, 11%), other pathogens (103, 22%)). Surgery was performed in 190 (40.2 %) patients. Follow-up clinical data showed that the 30-day and 1-year mortality due to infective endocarditis were 9.6% and 14%, respectively.
Discussion
The endocarditis Board is currently considered the cornerstone of decision-making in infective endocarditis [2,14,15,18,19,20,21,22,23,24,25,26,27,28]. An official Endocarditis Board meeting is held on a weekly basis at our institution. There is a structured protocol with documentation of pre- and post-discussion activities in the electronic medical record. This enhances the value of the joint decisions taken by the Endocarditis Board Team.
Our initial results correlate with current epidemiological studies, where NVIE continues to be the most common form of infective endocarditis [9]. We found almost equal PVIE and device-related infective endocarditis cases, pointing out the importance of changing epidemiology in this setting. Similar to the available literature, S. aureus was the most common causative pathogen [7,9] and surgery was required in more than 40% of cases [1,2,29].
Some sources in the literature have analysed the effects of Endocarditis Boards on patient outcomes [15,19,25,29] and seem to support an active role of these internal bodies in clinical practice. Although specific outcomes may be subject to institutional differences in patient referral and internal team factors, the role of the Endocarditis Board multidisciplinary team is being increasingly accepted, with more teams reporting institutional experiences, from organisational matters to clinical impact [2,14,15,18,19,20,21,22,23,24,25,26,27,28]. Of note is that individual physician perception of the Endocarditis Board multidisciplinary team is an important part of the topic, as the hospital physicians will be able to register patients online for the weekly meeting and assess the effect of discussions on outcomes. This has been recently addressed by El-Dalati et al. [28] supporting a wider adoption of the Endocarditis Board multidisciplinary team model.
Other aspects of the Endocarditis Board multidisciplinary team not addressed here may require further separate discussion. The educational role of the Endocarditis Board multidisciplinary team cannot be neglected. By pooling a number of different specialists together, patients enrolled in ENVALVE get educated during follow-up visits. Educational materials like individual departmental specific brochures, well drafted by the involved departments (Cardiology, Cardiac Surgery, Infectious Diseases) are given to patients on admission and after discharge. This contributes to create and reinforce awareness on the disease and its possible late consequences.
Research is another critical aspect of the Endocarditis Board. Data is collected and an institutional registry is maintained (ENVALVE). As briefly reported in the methods section, the existence of an approved protocol guarantees uniform collection of data aiming at evaluating pre-specified outcomes including mortality (early and late), the analysis of recurrences and relapses, surgical outcomes, etc. Our Institutional Registry can be used to determine the indications and optimal timings of different therapeutic approaches including surgery, antimicrobial therapy for complex infections and comparisons. Quality improvement, clinical and basic research are fundamental goals [30,31].
Limitations
This is a description of a local operational working scheme that may differ from other practices, although the Endocarditis Board is currently contemplated in practice guidelines. The referral patterns, considering the type of admitting institution and case-mix with high complexity, make our patient population skewed towards high-risk and mortality, and may not represent a regular practice. Furthermore, those shown here are preliminary and descriptive results that warrant more detailed prospective investigation.
Conclusion
The Endocarditis Board is currently an integrated part in the treatment of infective endocarditis and is the standard of care at our institution following international recommendations [12]. Current ESC European Practice Guidelines for the management of infective endocarditis [12] suggest the need of an Endocarditis Team in order to effectively treat this highly lethal disease. Multidisciplinary consensual decisions might also enable optimization of standards of care in vascular graft infections. Preliminary results are in line with current epidemiological studies.
Disclosure statement
No financial support and no other potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualisation: Mathias Van Hemelrijck, Alberto Weber, Barbara Hasse, Annelies S. Zinkernagel, Matthias Greutmann, Michelle Frank, Carlos A. Mestres; Critical revision: Alexander Breitenstein, Ronny R. Buechel, Peter Bode, Matthias Greutmann, Christiane Gruner, Frank Ruschitzka, Dominique Bettex, Felix Tanner, Thierry Carrel, Robert Bauernschmitt, Michelle Frank; Drafting and writing of the manuscript: Mathias Van Hemelrijck, Michelle Frank, Barbara Hasse, Carlos A. Mestres; Data collection and analysis: Mathias Van Hemelrijck, Adrian Schmid, David Siemer, Oscar Cuevas, Michelle Frank, Barbara Hasse, Carlos A. Mestres; Final approval: Mathias Van Hemelrijck, Annelies S. Zinkernagel, Alberto Weber, Matthias Greutmann, Michelle Frank, Barbara Hasse, Carlos A. Mestres
Abbreviations
| CT | Computeded tomography |
| ICU | Intensive care unit |
| NVIE | Native valve infective endocarditis |
| PET/CT | Positron emission tomography/computed tomography |
| PVIE | Prosthetic valve infective endocarditis |
| TOE | Transoesophageal echocardiography |
| TTE | Transthoracic echocardiography |
References
- Chirillo, F.; Scotton, P.; Rocco, F.; Rigoli, R.; Borsatto, F.; Pedrocco, A.; et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am. J. Cardiol. 2013, 112, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Chinchilla, F.; Sánchez-Espín, G.; Ruiz-Morales, J.; Rodríguez-Bailón, I.; Melero-Tejedor, J.M.; Ivanova-Georgieva, R.; et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev. Esp. Cardiol. Engl. Ed. 2014, 67, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Anguita, M.; Ruiz, M.; Castillo, J.C.; Delgado, M.; Mesa, D.; et al. Clinical features and changes in epidemiology of infective endocarditis on pacemaker devices over a 27-year period (1987–2013). Europace 2016, 18, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Hernandez-Meneses, M.; Téllez, A.; Pericàs, J.; Falces, C.; Tolosana, J.M.; et al. Hospital Clinic Infective Endocarditis Investigators. The Changing Epidemiology of Infective Endocarditis in the Twenty-First Century. Curr. Infect. Dis. Rep. 2017, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.A.; Johnson, C.O.; Colquhoun, S.M.; Karthikeyan, G.; Beaton, A.; Bukhman, G.; et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N. Engl. J. Med. 2017, 377, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Correa de Sa, D.D.; Tleyjeh, I.M.; Anavekar, N.S.; Schultz, J.C.; Thomas, J.M.; Lahr, B.D.; et al. Epidemiological trends of infective endocarditis: A population-based study in Olmsted County, Minnesota. Mayo Clin. Proc. 2010, 85, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Fowler VGJr Miro, J.M.; Hoen, B.; Cabell, C.H.; Abrutyn, E.; Rubinstein, E.; et al. ICE Investigators. Staphylococcus aureus endocarditis: A consequence of medical progress. JAMA 2005, 293, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; et al. International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, A.; Linke, A.; Latib, A.; Ihlemann, N.; Urena, M.; Walther, T.; et al. Association Between Transcatheter Aortic Valve Replacement and Subsequent Infective Endocarditis and In-Hospital Death. JAMA 2016, 316, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Durack, D.T.; Lukes, A.S.; Bright, D.K. Duke Endocarditis Service. New criteria for diagnosis of infective endocarditis: Utilization of specific echocardiographic findings. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Beurtheret, S.; Mancini, J.; Gariboldi, V.; Casalta, J.P.; Riberi, A.; et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: A propensity analysis. Eur. Heart J. 2011, 32, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.; Sandoe, J.; Ray, S.; Prendergast, B.; Taggart, D.; Westaby, S.; et al. The infective endocarditis team: Recommendations from an international working group. Heart 2014, 100, 524–527. [Google Scholar] [CrossRef]
- Mestres, C.A.; Paré, J.C.; Miró, J.M. Working Group on Infective Endocarditis of the Hospital Clínic de Barcelona. Organization and Functioning of a Multidisciplinary Team for the Diagnosis and Treatment of Infective Endocarditis: A 30-year Perspective (1985–2014). Rev. Esp. Cardiol. Engl. Ed. 2015, 68, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Cervera, C.; del Río, A.; García, L.; Sala, M.; Almela, M.; Moreno, A.; et al. Hospital Clinic Endocarditis Study Group. Efficacy and safety of outpatient parenteral antibiotic therapy for infective endocarditis: A ten-year prospective study. Enferm. Infecc. Microbiol. Clin. 2011, 29, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; Madsen, T.; Elming, H.; Jensen, K.T.; et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N. Engl. J. Med. 2019, 380, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.; Ray, S.; Prendergast, B.; Graham, T.; Campbell, B.; Greenhalgh, D.; et al. Standards for heart valve surgery in a ‘Heart Valve Centre of Excellence’. Open Heart 2015, 2, e000216. [Google Scholar] [CrossRef] [PubMed]
- Tornos, P. Infective endocarditis: A serious and rare condition that needs to be handled in experienced hospitals. Rev Esp Cardiol. 2005, 58, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, E.F.; Huang, G.; Aldea, G.; Koomalsingh, K.; Klein, J.W.; Dhanireddy, S.; et al. A Multidisciplinary Pathway for the Diagnosis and Treatment of Infectious Endocarditis. Crit. Pathw. Cardiol. 2020, 19, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Camou, F.; Dijos, M.; Barandon, L.; Cornolle, C.; Greib, C.; Laine, M.; et al. Management of infective endocarditis and multidisciplinary approach. Med. Mal. Infect. 2019, 49, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Hansen, M.S.; Cohen, G.; Boyle, K.; Yang, A.; Rishu, A.; et al. Case conferences for infective endocarditis: A quality improvement initiative. PLoS ONE 2018, 13, e0205528. [Google Scholar] [CrossRef] [PubMed]
- Harrak, S.; Doghmi, N.; Fellat, B.; Zarzur, J.; Cherti, M. Infective endocarditis in Morocco through the experience of a hospital department]. Ann. Cardiol. Angeiol. Paris 2019, 68, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, E.; Chirillo, F.; Moreo, A.; Graziosi, M.; De Michele, L.; Faggiano, P.; et al. SIECVI Task Force for the Management of Infective Endocarditis. Practical implementation of the Endocarditis Team in ‘functional’ reference centres: The Italian hospital network experience and recommendations of the Italian Society of Echocardiography and Cardiovascular Imaging. J. Cardiovasc. Med. Hagerstown 2019, 20, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Ruch, Y.; Mazzucotelli, J.P.; Lefebvre, F.; Martin, A.; Lefebvre, N.; Douiri, N.; et al. Impact of Setting up an “Endocarditis Team” on the Management of Infective Endocarditis. Open Forum Infect. Dis. 2019, 6, ofz308. [Google Scholar] [CrossRef] [PubMed]
- Davierwala, P.M.; Marin-Cuartas, M.; Misfeld, M.; Borger, M.A. The value of an “Endocarditis Team”. Ann. Cardiothorac. Surg. 2019, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Holte, E.; Dweck, M.R.; Marsan, N.A.; D’Andrea, A.; Manka, R.; Stankovic, I.; et al. EACVI survey on the evaluation of infective endocarditis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 828–832. [Google Scholar] [CrossRef] [PubMed]
- El-Dalati, S.; Khurana, I.; Soper, N.; Cronin, D.; Shea, M.; Weinberg, R.L.; et al. Physician perceptions of a multidisciplinary endocarditis team. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Anguita Sánchez, M.; Torres Calvo, F.; Castillo Domínguez, J.C.; Delgado Ortega, M.; Mesa Rubio, D.; Ruiz Ortiz, M.; et al. Short- and long-term prognosis of infective endocarditis in non-injection drug users: Improved results over 15 years (1987–2001). Rev. Esp. Cardiol. 2005, 58, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, T.A.; Shambat, S.M.; Haunreiter, V.D.; Mestres, C.A.; Weber, A.; Maisano, F.; et al. Polyester Vascular Grant Material and Risk for Intracavitary Thoracic Vascular Graft Infection. Emerg. Infect. Dis. 2020, 26, 2448–2452. [Google Scholar] [CrossRef] [PubMed]
- Hasse, B.; Hannan, M.M.; Keller, P.M.; Maurer, F.P.; Sommerstein, R.; Mertz, D.M.; et al. chimaera ISCVID Investigators and; ISCVID Executive Committee; Infectious Diseases Specialists; Hospital Epidemiologists; Microbiologists and Molecular Typing Specialists; Cardiac Surgeons/ Perfusionists/ Cardiologists; Ophthalmology; Anaesthesiologists; Public Health. International Society of Cardiovascular Infectious Diseases Guidelines for the Diagnosis, Treatment and Prevention of Disseminated Mycobacterium chimaera Infection Following Cardiac Surgery with Cardiopulmonary Bypass. J. Hosp. Infect. 2020, 104, 214–235. [Google Scholar] [CrossRef] [PubMed]
© 2022 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.