SGTL-2 Inhibitors and Heart Failure
Summary
Introduction
Mechanisms
SGLT2i and heart failure prevention
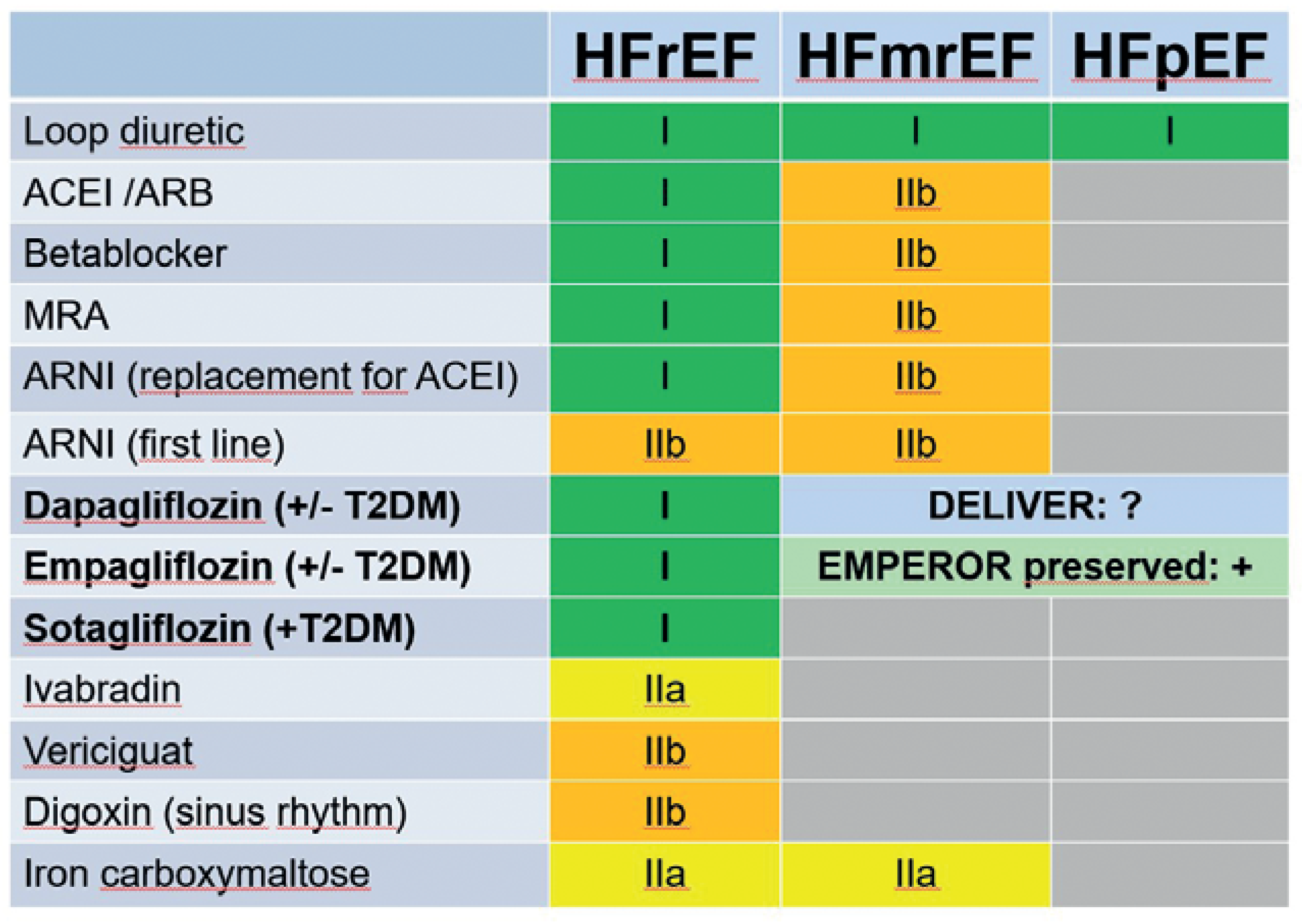
Heart failure with reduced ejection fraction
Heart failure with preserved ejection fraction
Disclosure statement
References
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020, 75, 422–34. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; McMurray, J.J. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018, 61, 2108–17. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Differential Pathophysiological Mechanisms in Heart Failure With a Reduced or Preserved Ejection Fraction in Diabetes. JACC Heart Fail. 2021, 9, 535–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Brooksbank, K.J.; Wetherall, K.; Mangion, K.; Roditi, G.; Campbell, R.T.; et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation 2021, 143, 516–25. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, et al; EMPA-TROPISM (ATRU-4) Investigators. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2021, 77, 243–55. [CrossRef] [PubMed]
- Requena-Ibáñez, J.A.; Santos-Gallego, C.G.; Rodriguez-Cordero, A.; Vargas-Delgado, A.P.; Mancini, D.; Sartori, S.; et al. Mechanistic Insights of Empagliflozin in Nondiabetic Patients With HFrEF: from the EMPA-TROPISM Study. JACC Heart Fail. 2021, 9, 578–89. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Jensen, J.; Ali, M.; Frederiksen, P.H.; Kistorp, C.; Videbæk, L.; et al. Associations of Empagliflozin With Left Ventricular Volumes, Mass, and Function in Patients With Heart Failure and Reduced Ejection Fraction: A Substudy of the Empire HF Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 836–40. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Jensen, J.; Frederiksen, P.H.; Kistorp, C.; Videbæk, L.; Poulsen, M.K.; et al. Effect of Empagliflozin on Hemodynamics in Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2020, 76, 2740–51. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Omar, M.; Kistorp, C.; Poulsen, M.K.; Tuxen, C.; Gustafsson, I.; et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: A double-blinded, randomized, and placebo-controlled trial. Am Heart J. 2020, 228, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Martens, P.; Forouzan, O.; Dauw, J.; Vercammen, J.; Luwel, E.; et al. Effects of dapagliflozin on congestion assessed by remote pulmonary artery pressure monitoring. ESC Heart Fail. 2020, 7, 2071–3. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Gandy, S.; McCrimmon, R.; Houston, J.G.; Struthers, A.D.; Lang, C.C. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J. 2020, 41, 3421–32. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Gandy, S.; Mordi, I.R.; McCrimmon, R.; Ramkumar, P.G.; Houston, J.G.; et al. Dapagliflozin Improves Left Ventricular Myocardial Longitudinal Function in Patients With Type 2 Diabetes. JACC Cardiovasc Imaging 2021, 14, 503–4. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; Qintar, M.; Windsor, S.L.; Jermyn, R.; Shavelle, D.M.; Tang, F.; et al. Empagliflozin Effects on Pulmonary Artery Pressure in Patients With Heart Failure: Results From the EMBRACE-HF Trial. Circulation. 2021, 143, 1673–86. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 2019, 140, 1693–702. [Google Scholar] [CrossRef] [PubMed]
- Mazer, C.D.; Hare, G.M.; Connelly, P.W.; Gilbert, R.E.; Shehata, N.; Quan, A.; et al. Effect of Empagliflozin on Erythropoietin Levels, Iron Stores, and Red Blood Cell Morphology in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease. Circulation. 2020, 141, 704–7. [Google Scholar] [CrossRef] [PubMed]
- Sarak, B.; Verma, S.; David Mazer, C.; Teoh, H.; Quan, A.; Gilbert, R.E.; et al. Impact of empagliflozin on right ventricular parameters and function among patients with type 2 diabetes. Cardiovasc Diabetol. 2021, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.; Coelho-Filho, O.R.; Verma, S.; Chowdhury, B.; Zuo, F.; Quan, A.; et al. Empagliflozin Reduces Myocardial Extracellular Volume in Patients With Type 2 Diabetes and Coronary Artery Disease. JACC Cardiovasc Imaging. 2021, 14, 1164–73. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Martens, P. Empagliflozin-Induced Changes in Epicardial Fat: The Centerpiece for Myocardial Protection? JACC Heart Fail. 2021, 9, 590–3. [Google Scholar] [CrossRef] [PubMed]
- McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019, 381, 1995–2008. [CrossRef] [PubMed]
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020, 383, 1413–24. [CrossRef] [PubMed]
- Januzzi JL Jr, Zannad F, Anker SD, Butler J, Filippatos G, Pocock SJ, et al; EMPEROR-Reduced Trial Committees and Investigators. Prognostic Importance of NT-proBNP and Effect of Empagliflozin in the EMPEROR-Reduced Trial. J Am Coll Cardiol. 2021, 78, 1321–32. [CrossRef] [PubMed]
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021, 385, 1451–61. [CrossRef] [PubMed]
- Mullens, W.; Martens, P. Empagliflozin and renal sodium handling: an intriguing smart osmotic diuretic. Eur J Heart Fail. 2021, 23, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Rao, V.S.; Ivey-Miranda, J.; Fleming, J.; Mahoney, D.; Maulion, C.; et al. Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects. Circulation. 2020, 142, 1028–39. [Google Scholar] [CrossRef] [PubMed]
- Mordi, N.A.; Mordi, I.R.; Singh, J.S.; McCrimmon, R.J.; Struthers, A.D.; Lang, C.C. Renal and Cardiovascular Effects of SGLT2 Inhibition in Combination With Loop Diuretics in Patients With Type 2 Diabetes and Chronic Heart Failure: the RECEDE-CHF Trial. Circulation 2020, 142, 1713–24. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, E.M.; Beusekamp, J.C.; Ter Maaten, J.M.; Figarska, S.M.; Danser, A.H.; van Veldhuisen, D.J.; et al. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail. 2021, 23, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Grodin, J.L.; Tang, W.H. Sodium-Glucose Cotransporter-2 Inhibitors and Loop Diuretics for Heart Failure: Priming the Natriuretic and Metabolic Reserve of the Kidney. Circulation. 2020, 142, 1055–8. [Google Scholar] [CrossRef] [PubMed]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015, 373, 2117–28. [CrossRef] [PubMed]
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017, 377, 644–57. [CrossRef] [PubMed]
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, et al; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019, 380, 2295–306. [CrossRef] [PubMed]
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019, 380, 347–57. [CrossRef] [PubMed]
- Cosentino F, Cannon CP, Cherney DZ, Masiukiewicz U, Pratley R, Dagogo-Jack S, et al; VERTIS CV Investigators. Efficacy of Ertugliflozin on Heart Failure-Related Events in Patients With Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease: results of the VERTIS CV Trial. Circulation 2020, 142, 2205–15. [CrossRef] [PubMed]
- Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al; SCORED Investigators. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 2021, 384, 129–39. [CrossRef] [PubMed]
- Kato, E.T.; Silverman, M.G.; Mosenzon, O.; Zelniker, T.A.; Cahn, A.; Furtado, R.H.; et al. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation. 2019, 139, 2528–36. [Google Scholar] [CrossRef] [PubMed]
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESCScientific Document Group 2021 ESCGuidelines for the diagnosis treatment of acute chronic heart failure. Eur Heart J. 2021, 42, 3599–726. [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020, 396, 819–29. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Butler, J. Victims of Success in Failure. Circulation. 2020, 142, 1129–31. [Google Scholar] [CrossRef] [PubMed]
- Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, et al.; EMPEROR-Reduced Trial Committees Investigators Influence of neprilysin inhibition on the efficacy safety of empagliflozin in patients with chronic heart failure a reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021, 42, 671–80. [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Fonarow, G.C. Simultaneous or Rapid Sequence Initiation of Quadruple Medical Therapy for Heart Failure-Optimizing Therapy With the Need for Speed. JAMA Cardiol. 2021, 6, 743–4. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Dewan, P.; Anand, I.S.; Bělohlávek, J.; Bengtsson, O.; de Boer, R.A.; et al. Dapagliflozin and Diuretic Use in Patients With Heart Failure and Reduced Ejection Fraction in DAPA-HF. Circulation. 2020, 142, 1040–54. [Google Scholar] [CrossRef] [PubMed]
- Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al.; SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021, 384, 117–28. [PubMed]
- Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al.; EMPEROR-Preserved Trial Committees and Investigators. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail. 2019, 21, 1279–87. [PubMed]
- Solomon, S.D.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021, 23, 1217–25. [Google Scholar] [CrossRef] [PubMed]
- https://www.ajmc.
- Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al.; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003, 362, 777–81. [PubMed]
- Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014, 370, 1383–92. [PubMed]
- Solomon SD, McMurray JJ, Anand IS, Ge J, Lam CS, Maggioni AP, et al; PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019, 381, 1609–20. [PubMed]
- Packer M, Butler J, Zannad F, Pocock SJ, Filippatos G, Ferreira JP, et al; EMPEROR Study Group. Empagliflozin and Major Renal Outcomes in Heart Failure. N Engl J Med. 2021, 385, 1531–3. [PubMed]
- https://www.radcliffecardiology.com/news/preserved-hf-trial-demonstrates-substantial-improvements-symptoms-physical-limitations-and.
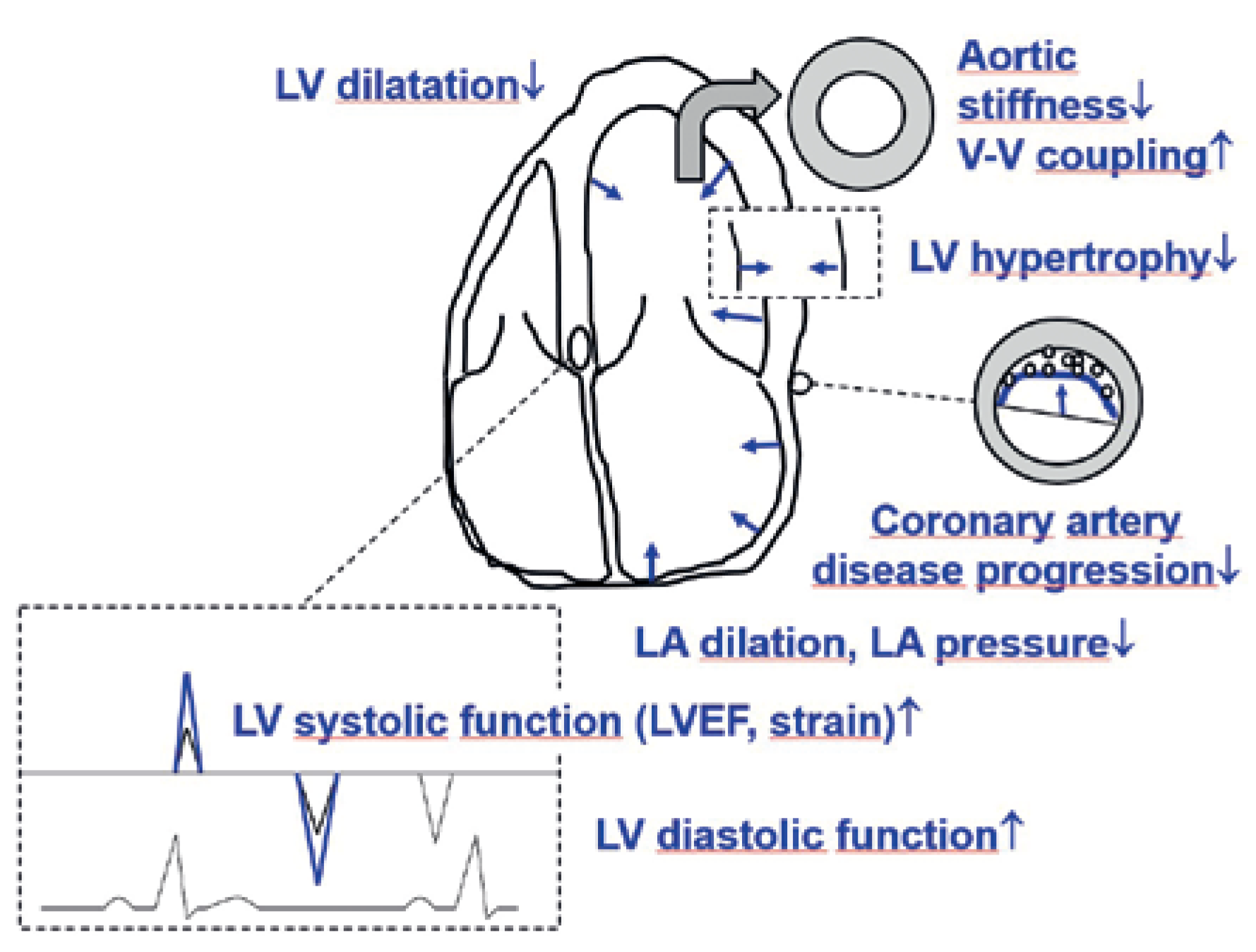
| Diuretic effect (synergistic with loop diuretic) |
| Cross-reaction with the cardiac sodium-hydrogen exchanger 1 with increase in mitochondrial calcium and improved cardiac function |
| Switch of myocardial substrate utilisation from glucose toward free fatty acids, ketone bodies, and branched amino acids |
| Lipolysis and reduction of pericardial adipose tissue with attenuation of adipokine signalling and thereby attenuation of pro-inflammatory and pro-fibrotic mechanisms |
| Improved vascular function with improved ventriculo-vascular coupling |
| Erythropoietin stimulation |
| Study population | Intervention/investigation | Main findings | |
| Reduced LVEF | |||
| Lee et al. 2021 [4] | 105 patients with HFreF, mean LVEF 33% (all ≤40%), T2DM/prediabetes, median Nt-proBNP 466 ng/l | randomisation to eMPa 10 mg versus placebo for 36 weeks; assessment of lv volumes by cardiac Mri | reduction in lv end-systolic and end- diastolic volume index as well as NtproBNP, but not global longitudinal strain and LVEF, with eMPa |
| Santos-Gallego et al. 2021 [5] | 84 patients with HFrEF, LVE F 36%, (all ≤50%), no diabetes | randomisation to eMPa 10 mg versus placebo for 6 months; assessment of lv volumes by cardiac Mri | reduction in lv end-diastolic and end- systolic volume and lv mass, and increase in LVEF, peak oxygen consumption and 6 minute walking distance with eMPa |
| Requena-Ibanez et al. 2021 [6] | 84 patients with HFrEF (all ≤50%), no diabetes | randomisation to eMPa 10 mg versus placebo for 6 months; assessment by cardiac Mri | reduction in epicardial adipose tissue, subcutaneous adipose tissue, extracellular volume, matrix volume, cardiomyocyte volume and aortic stiffness with eMPa |
| Jensen et al. 2020 [9] | 70 patients with HFreF (LVEF ≤40%) with or without T2DM | randomisation to eMPa 10 mg versus placebo for 12 weeks. Measurement of NtproBNP at baseline and after 12 weeks. | No effect on Nt-proBNP |
| Omar et al. 2020 [8] | 70 patients with HFreF (LVEF ≤40%) with or without T2DM | randomisation to eMPa 10 mg versus placebo for 12 weeks. exercise right heart catheterisation at baseline and after 12 weeks. | reduction in mean pulmonary artery wedge pressure during exercise with eMPa, no effect on cardiac index |
| Omar et al. 2021 [7] | 186 patients with HFreF (LVEF ≤40%) with or without T2DM | randomszation to eMPa 10 mg versus placebo for 12 weeks. echocardiography at baseline and after 12 weeks. | reduction in lv end-diastolic and end- systolic volume index and left atrial volume index with eMPa, no effect on LVEF |
| Mullens et al. 2020 [10] | 9 patients with HFreF | treatment with daPa, no control group; all patients had an implanted PaP sensor | reduction in mean PaP from 42 to 38 mm Hg within 7 days |
| Preserved LVEF | |||
| Brown et al. 2020 [11] | 66 patients, T2DM, no HF, lv hypertrophy (lv mass index >115 g/m2 in men and >95 g/m2 in women), good blood pressure control (<145/90 mm Hg) | randomisation to daPa 10 mg versus placebo for 12 months; assessment of lv mass by cardiac Mri | reduction in lv mass with daPa; reduction in systolic blood pressure, body weight, adipose tissue and insulin resistance |
| Brown et al 2021 [12] | 47 patients, T2DM, no HF, lv hypertrophy (lv mass index >115 g/m2 in men and >95 g/m2 in women), good blood pressure control (<145/90 mm Hg) | randomisation to daPa 10 mg versus placebo for 12 months; assessment of lv global longitudinal strain by echocardiography | improvement in global longitudinal strain with daPa, no effect on e’ and e/e’ |
| Reduced or preserved LVEF | |||
| Nassif et al. 2021 [13] | 65 patients with HF: LVEF 44%, 52% T2DM, median Nt-proBNP 637 ng/l | randomisation to eMPa 10 mg versus placebo for 12 weeks; all patients had an implanted PaP sensor | Baseline diastolic PaP 22 mm Hg; at 12 weeks: diastolic PaP 1.7 mm Hg lower in eMPa group despite absence of a difference in loop diuretic dose |
| verma et al. 2019 [14] | 90 patients with coronary artery disease and T2DM, mean LVEF ≈57% (all ≥30%), most without HF | randomisation to eMPa 10 mg versus placebo for 6 months; assessment by cardiac Mri | reduction in lv mass index and systolic and diastolic blood pressure, and increase in haematocrit with eMPa |
| Mason et al. 2021 [17] | 74 patients with coronary artery disease and T2DM, LVEF ≈57% (all ≥30%), most without HF | randomisation to eMPa 10 mg versus placebo for 6 months; assessment by cardiac Mri | reduction in extracellular volume with eMPa |
| Mazer et al. 2019 [15] | 80 patients with coronary artery disease and T2DM, LVEF mainly >50% (all ≥30%), most without HF | randomisation to eMPa 10 mg versus placebo for 6 months, blood samples at baseline, 1 month, and 6 months | increase in erythropoietin at 1 month (not significant at 6 months) and haemoglobin, and decrease in ferritin with eMPa |
| Sarak et al. 2021 [16] | 90 patients with coronary artery d isease and T2DM, LVEF mainly ≈57% (all ≥30%), most without HF | randomisation to eMPa 10 mg versus placebo for 6 months; assessment by cardiac Mri | No effect by eMPa on RV mass, RV volumes and RV ejection fraction |
| DAPA-HF | eMPeROR reduced | |||
| Dapagliflozin (n = 2373) | Placebo (n = 2371) | empagliflozin (n = 1863) | Placebo (n = 1867) | |
| Baseline characteristics | ||||
| age (years) | 66 ± 11 | 67 ± 11 | 67 ± 11 | 67 ± 11 |
| Female sex (%) | 24 | 23 | 24 | 24 |
| Body mass index (kg/m2) | 28 ± 6 | 28 ± 6 | 28 ± 6 | 28 ± 5 |
| diabetes (%) | 42 | 42 | 50 | 50 |
| LVEF (%) | 31 ± 7 | 31 ± 7 | 28 ± 6 | 27 ± 6 |
| Nt-proBNP (ng/l) | 1428 (857–2655) | 1446 (857–2641) | 1887 (1077–3429) | 1926 (1153–3525) |
| Systolic blood pressure (mm Hg) | 122 ± 16 | 122 ± 16 | 123 ± 16 | 121 ± 15 |
| atrial fibrillation (%) | 39 | 38 | 36 | 38 |
| eGFr (ml/min/1.73 m2) | 66 ± 20 | 66 ± 19 | 62 ± 22 | 62 ± 22 |
| Baseline therapy | ||||
| diuretic (%) | 93 | 94 | Na§ | Na§ |
| aCei (%) | 56 | 56 | 47 | 45 |
| arB (%) | 28 | 27 | 24 | 25 |
| arNi (%) | 11 | 11 | 18 | 21 |
| Beta-blocker (%) | 96 | 96 | 95 | 95 |
| Mra (%) | 72 | 71 | 70 | 73 |
| digitalis (%) | 19 | 19 | Na | Na |
| Cardiac resynchronisation therapy (%) | 8 | 7 | 12 | 12 |
| defibrillator (%) | 26 | 26 | 31 | 32 |
| Study endpoints | ||||
| Primary endpoint* | ||||
| – % | 16.3 | 21.2 | 19.4 | 24.7 |
| – events/100 patients years | 11.6 | 15.6 | 15.8 | 21.0 |
| – Hr (95% Ci) | 0.74 (0.65–0.85) | 0.75 (0.65–0.86) | ||
| – Number needed to treat | 21 (18 months) | 19 (16 months) | ||
| Cardiovascular death | ||||
| – % | 9.6 | 11.5 | 10.0 | 10.8 |
| – events/100 patients years | 6.5 | 7.9 | 7.6 | 8.1 |
| – Hr (95% Ci) | 0.82 (0.69–0.98) | 0.92 (0.75–1.12) | ||
| HF hospitalisations | ||||
| – % | 9.7 | 13.4 | 13.2 | 18.3 |
| – events/100 patients years | 6.9 | 9.8 | 10.7 | 15.5 |
| – Hr (95% Ci) | 0.70 (0.59–0.83) | 0.69 (0.59–0.81) |
© 2022 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Maeder, M.T.; Rickli, H.; Buser, M. SGTL-2 Inhibitors and Heart Failure. Cardiovasc. Med. 2022, 25, 36. https://doi.org/10.4414/cvm.2022.02206
Maeder MT, Rickli H, Buser M. SGTL-2 Inhibitors and Heart Failure. Cardiovascular Medicine. 2022; 25(2):36. https://doi.org/10.4414/cvm.2022.02206
Chicago/Turabian StyleMaeder, Micha T., Hans Rickli, and Marc Buser. 2022. "SGTL-2 Inhibitors and Heart Failure" Cardiovascular Medicine 25, no. 2: 36. https://doi.org/10.4414/cvm.2022.02206
APA StyleMaeder, M. T., Rickli, H., & Buser, M. (2022). SGTL-2 Inhibitors and Heart Failure. Cardiovascular Medicine, 25(2), 36. https://doi.org/10.4414/cvm.2022.02206




