Detection and Management of Subclinical Atrial Fibrillation in Implantable and Wearable Devices
Abstract
Introduction
Screening for AF?
AF detection using cardiac implantable electronic devices
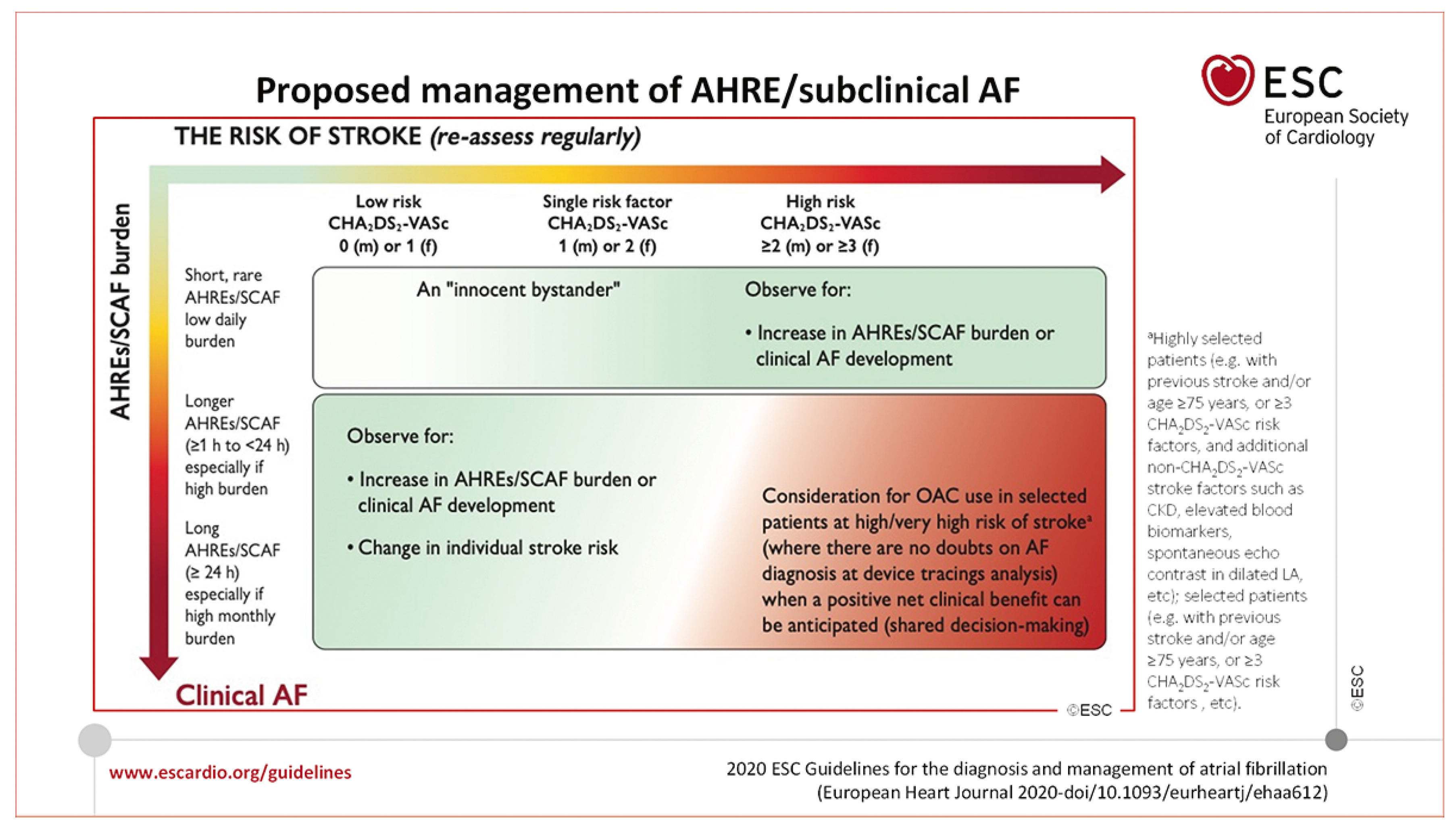
Management of subclinical AF
AF detection in wearable devices
Outlook
Key points
Disclosure statement
References
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987, 147, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Hart, C.L.; Hole, D.J.; McMurray, J.J. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002, 113, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Wattigney, W.A.; Mensah, G.A.; Croft, J.B. Increased atrial fibrillation mortality: united States, 1980-1998. Am J Epidemiol. 2002, 155, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.M.; Kleinig, T.J.; Newbury, J.; Castle, S.; Cranefield, J.; Anderson, C.S.; et al. Adelaide stroke incidence study: declining stroke rates but many preventable cardioembolic strokes. Stroke. 2013, 44, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.; Rosenqvist, M.; Lindgren, A.; Terént, A.; Norrving, B.; Asplund, K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014, 45, 2599–2605. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Perera, T.; Elliott, A.D.; Twomey, D.J.; Kumar, S.; Munwar, D.A.; et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018, 39, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; BlomströmLundqvist, C.; et al.; ESC Scientific Document Group 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Glotzer, T.V.; Hellkamp, A.S.; Zimmerman, J.; Sweeney, M.O.; Yee, R.; Marinchak, R.; et al.; MOST Investigators Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003, 107, 1614–1619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; et al.; ASSERT Investigators Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012, 366, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Lip, G.Y.; De Caterina, R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; et al.; ESC Committee for Practice Guidelines-CPG; Document Reviewers 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012, 14, 1385–1413. [Google Scholar] [CrossRef] [PubMed]
- Mairesse, G.H.; Moran, P.; Van Gelder, I.C.; Elsner, C.; Rosenqvist, M.; Mant, J.; et al.; ESC Scientific Document Group Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace. 2017, 19, 1589–1623. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; et al.; EMBRACE Investigators and Coordinators Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Sanna, T.; Diener, H.C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; et al.; CRYSTAL AF Investigators Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Ciconte, G.; Giacopelli, D.; Pappone, C. The Role of Implantable Cardiac Monitors in Atrial Fibrillation Management. J Atr Fibrillation. 2017, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Alings, M.; Ha, A.; Leong-Sit, P.; Birnie, D.H.; de Graaf, J.J.; et al.; ASSERT-II Investigators Subclinical Atrial Fibrillation in Older Patients. Circulation. 2017, 136, 1276–1283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glotzer, T.V.; Daoud, E.G.; Wyse, D.G.; Singer, D.E.; Ezekowitz, M.D.; Hilker, C.; et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009, 2, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Quirino, G.; Giammaria, M.; Corbucci, G.; Pistelli, P.; Turri, E.; Mazza, A.; et al. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009, 32, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Swiryn, S.; Orlov, M.V.; Benditt, D.G.; DiMarco, J.P.; Lloyd-Jones, D.M.; Karst, E.; et al.; RATE Registry Investigators Clinical Implications of Brief Device-Detected Atrial Tachyarrhythmias in a Cardiac Rhythm Management Device Population: Results from the Registry of Atrial Tachycardia and Atrial Fibrillation Episodes. Circulation. 2016, 134, 1130–1140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Botto, G.L.; Padeletti, L.; Santini, M.; Capucci, A.; Gulizia, M.; Zolezzi, F.; et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009, 20, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Pettorelli, D. Atrial fibrillation burden and atrial fibrillation type: clinical significance and impact on the risk of stroke and decision making for long-term anticoagulation. Vascul Pharmacol. 2016, 83, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Brambatti, M.; Connolly, S.J.; Gold, M.R.; Morillo, C.A.; Capucci, A.; Muto, C.; Lau, C.P.; Van Gelder, I.C.; Hohnloser, S.H.; Carlson, M.; Fain, E.; Nakamya, J.; Mairesse, G.H.; Halytska, M.; Deng, W.Q.; Israel, C.W.; Healey, J.S. on behalf of the ASSERT Investigators. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014, 129, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.C.; Fan, J.; Askari, M.; Heidenreich, P.A.; Keung, E.; Raitt, M.H.; et al. Practice Variation in Anticoagulation Prescription and Outcomes After Device-Detected Atrial Fibrillation. Circulation. 2019, 139, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Keating, R.J.; Markowitz, S.M.; Liu, C.F.; Thomas, G.; Ip, J.E.; et al. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm. 2014, 11, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.T.; Kronborg, M.B.; Nohr, E.A.; Mortensen, P.T.; Gerdes, C.; Nielsen, J.C. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Heart Rhythm. 2015, 12, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Healey, J.S.; Crijns, H.J.; Wang, J.; Hohnloser, S.H.; Gold, M.R.; et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017, 38, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Kamišalić, A.; Fister, I.J.r.; Turkanović, M.; Karakatič, S. Sensors and Functionalities of Non-Invasive Wrist-Wearable Devices: A Review. Sensors 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Eerikäinen, L.M.; Bonomi, A.G.; Dekker, L.R.; Vullings, R.; Aarts, R.M. Atrial fibrillation monitoring with wrist-worn photoplethysmographybased wearables: state-of-the-art review. Cardiovascular Digital Health Journal. 2020, 1, 45–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; et al.; Apple Heart Study Investigators Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, H.; Zhang, H.; Liu, T.; Liang, Z.; Xia, Y.; et al.; MAFA II Investigators Mobile Photoplethysmographic Technology to Detect Atrial Fibrillation. J Am Coll Cardiol. 2019, 74, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.; Ding, W.Y.; Etheridge, S.; Noseworthy, P.A.; Veltmann, C.; Yao, X.; et al. Smart Wearables for Cardiac Monitoring-Real-World Use beyond Atrial Fibrillation. Sensors 2021, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Blank, B.F.; Calvert, M.; Camm, A.J.; Chlouverakis, G.; Diener, H.C.; et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAHAFNET 6) trial. Am Heart J. 2017, 190, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Alings, M.; Connolly, S.J.; Beresh, H.; Granger, C.B.; Mazuecos, J.B.; et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected SubClinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017, 189, 137–145. [Google Scholar] [CrossRef] [PubMed]
© 2021 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Berg, J.; Haegeli, L.M. Detection and Management of Subclinical Atrial Fibrillation in Implantable and Wearable Devices. Cardiovasc. Med. 2021, 24, w10101. https://doi.org/10.4414/cvm.2021.02180
Berg J, Haegeli LM. Detection and Management of Subclinical Atrial Fibrillation in Implantable and Wearable Devices. Cardiovascular Medicine. 2021; 24(6):w10101. https://doi.org/10.4414/cvm.2021.02180
Chicago/Turabian StyleBerg, Jan, and Laurent M Haegeli. 2021. "Detection and Management of Subclinical Atrial Fibrillation in Implantable and Wearable Devices" Cardiovascular Medicine 24, no. 6: w10101. https://doi.org/10.4414/cvm.2021.02180
APA StyleBerg, J., & Haegeli, L. M. (2021). Detection and Management of Subclinical Atrial Fibrillation in Implantable and Wearable Devices. Cardiovascular Medicine, 24(6), w10101. https://doi.org/10.4414/cvm.2021.02180



