Introduction
Acute coronary syndrome (ACS) is known to be one of the major causes of mortality and morbidity in the western world [1]. Recent official data in Switzerland show that ACS is responsible for about 30% of deaths and 14% of hospitalisations in men, and about 34% and 9%, respectively, in women [2].
Timely primary percutaneous coronary intervention (pPCI) greatly reduces morbidity and mortality of ACS patients [3,4]. One of the most important complications of PCI is thrombosis of the stent, leading to complete occlusion of the culprit coronary artery. Stent thrombosis is reported to occur in about 1–2% of patients with stent insertion because of either ACS or unstable or stable angina pectoris [5,6].
Many risk factors for stent thrombosis have been described, with early discontinuation of dual antiplatelet therapy (DAPT), stenting in patients with ST-elevation myocardial infarction (STEMI), patient history of stent thrombosis and small target vessel/stent diameter being among the ones most often identified [7,8]. Bare metal stents (BMSs) and drug eluting stents (DESs) are known to show different risk profiles for stent thrombosis [9,10]. First-generation DESs (sirolimusand- paclitaxel-eluting stents) were at the highest risk of developing late stent thrombosis (i.e., more than 30 days after stenting); the risk dramatically decreased with second-generation DESs (zotarolimusand everolimuseluting stents) [11]. Large-scale trials showed lower rates of stent thrombosis and revascularisation for secondgeneration DESs compared with BMSs [10,12].
Many international multicentre studies on stent thrombosis have been published, but differences in healthcare systems, patient demographics and cultural factors are well-known to result in differences in its occurrence in daily patient care [13].
In this study, we analysed data from the Special Programme University Medicine study (SPUM-ACS) on stent thrombosis and compared them with important studies and data from large-scale registries published in the literature.
Methods
Study population
The prospective multicentre Special Programme University Medicine (SPUM) – ACS Biomarker cohort [14,15] recruited patients who were referred for coronary angiography with the diagnosis of ACS to one of the participating Swiss university hospitals (Zurich, Bern, Lausanne and Geneva) between December 2009 and October 2012. It featured consecutive patient recruitment, with follow-up at 30 days (telephone call) and 1 year (clinical visit). Female and male patients aged 18 years or older presenting within 5 days (preferably within 72 hours) after pain onset with the main diagnosis of STEMI, non-ST-elevation myocardial infarction (NSTEMI) or unstable angina were included. Within the consortium, a centralised electronic database provided comprehensive information on all patients. All adverse events occurring within 1 year after the index ACS event were ascertained at 30 days (telephone call) and 1 year (clinical visit) and adjudicated by an independent committee consisting of three experienced cardiologists (Lukas Kappenberger, Lausanne; Tiziano Moccetti, Lugano; Mathias E. Pfisterer, Basel).
Patient selection
Included patients had symptoms compatible with angina pectoris (chest pain, dyspnoea) and fulfilled at least one of the following criteria: (a) ECG changes such as persistent ST segment elevation or depression, T wave inversion or dynamic ECG changes, new left bundle-branch block; (b) evidence of positive (predominantly conventional) troponin according to local laboratory reference values; (c) known coronary artery disease, specified as status after myocardial infarction, or PCI or newly documented ≥50% stenosis of an epicardial coronary artery during the initial catheterisation. Exclusion criteria included severe physical disability, inability to comprehend study and less than 1 year of life expectancy for noncardiac reasons.
Medications
The protocol for medications during the hospital stay and follow-up was determined by consensus a priori. It comprised administration of aspirin and an additional platelet inhibitor (prasugrel, ticagrelor or clopidogrel) with prasugrel preferred for STEMI patients (loading dose of 60 mg, followed by 10 mg/d) and clopidogrel for NSTEMI patients (loading dose of 300 mg, followed by 75 mg/d) for 1 year after the ACS unless an indication for oral anticoagulation was present. Treating physicians were advised to administer a statin (rosuvastatin 20 mg/d), an angiotensin converting-enzyme inhibitor or angiotensin II receptor blocker, and a beta-blocker as soon after the ACS as they could be tolerated by the patient.
Heparin was routinely administered at angiography in all centres, but the dose was left to the discretion of the interventional cardiologist. Glycoprotein IIb/IIIa inhibitors were rarely used.
Laboratory testing
Routine tests were performed at laboratories of each institution. Because different assays were used for troponin, ratios of the result to the upper limit of the reference range were reported. Creatinine clearance was estimated with use of the Cockcroft–Gault equation in the current analysis.
Definitions
Stent thrombosis comprised either complete or partial stent occlusion as seen in angiography (angiographic findings were interpreted as described in the original report). An event occurring within the first 30 days of stent implantation was defined as early (sub-acute) stent thrombosis, whereas those occurring between 30 days and 1 year were considered late stent thrombosis. Cardiac death was defined as any death due to a proximate cardiac cause (e.g., myocardial infarction [MI], low-output failure, fatal arrhythmia), unwitnessed death and death of unknown cause, and all procedurerelated deaths including those related to concomitant treatment were classified as cardiac death by the adjudication committee. Major adverse cardiac and cardiovascular events (MACCE) were defined in this analysis as a composite of all-cause mortality, cerebrovascular event, any repeat revascularisation and MI. Major adverse cardiac events (MACE) were defined as a composite of cardiac death, clinically indicated revascularisation and MI [16].
The presence of risk factors was determined either on the basis of the patient’s history and drug use (many patients were taking cardiovascular drugs such as aspirin and statins, as well as antihypertensives and antidiabetics) or on the diagnosis made during hospitalisation, according to published European Society of Cariology (ESC) guidelines [17,18].
Primary outcome
The primary outcome in our study was stent thrombosis at 1-year follow-up.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median with interquartile range (IQR), or median and 25th/75th interquartile range for skewed variables. They were compared using one-way analysis of variance (ANOVA), student’s t-test or the Mann Whitney U-test as appropriate. Categorical data are presented as frequencies (percentages) and were compared using the Fisher exact or the chi-square tests.
All p-values and confidence intervals were two-sided. A p-value of <0.05 was considered significant, and all tests were two-tailed. All analyses were performed with SPSS version 21.0 software (SPSS Inc., Chicago, Ill).
Discussion
In this large prospective Swiss real-world ACS cohort referred for primary PCI for ACS we found a very low rate of stent thrombosis of 1.1%. The low rate of stent thrombosis is well in line with the findings of other “realworld” registries, such as the large Swedish SCAAR registry with a stent thrombosis rate of 1.2% [5]. Stent thrombosis risk was lower in a DES than in a BMS, which matches the results of a recent meta-analysis [19]. As patients with ACS, particularly those with STEMI, have a large thrombus burden at the site of coronary narrowing or occlusion, the risk of stent thrombosis was initially considered much higher than in patients with stable angina undergoing elective PCI. The very low stent thrombosis rate of 1.1% reflects the impressive progress that has been achieved over recent decades in stent design and implantation technique. The higher risk of stent thrombosis in patients presenting with STEMI is known and reflects the importance of intracoronary thrombus load for the development of such an event [20]. In STEMI patients platelet activity is markedly elevated and endothelial healing delayed, thereby exposing naked thrombogenic stent struts to the circulating blood [21]. Of note, patients with stent thrombosis had been implanted with stents 20 mm in length, which may have contributed to delayed or incomplete healing, which in turn may have increased the risk of such an event.
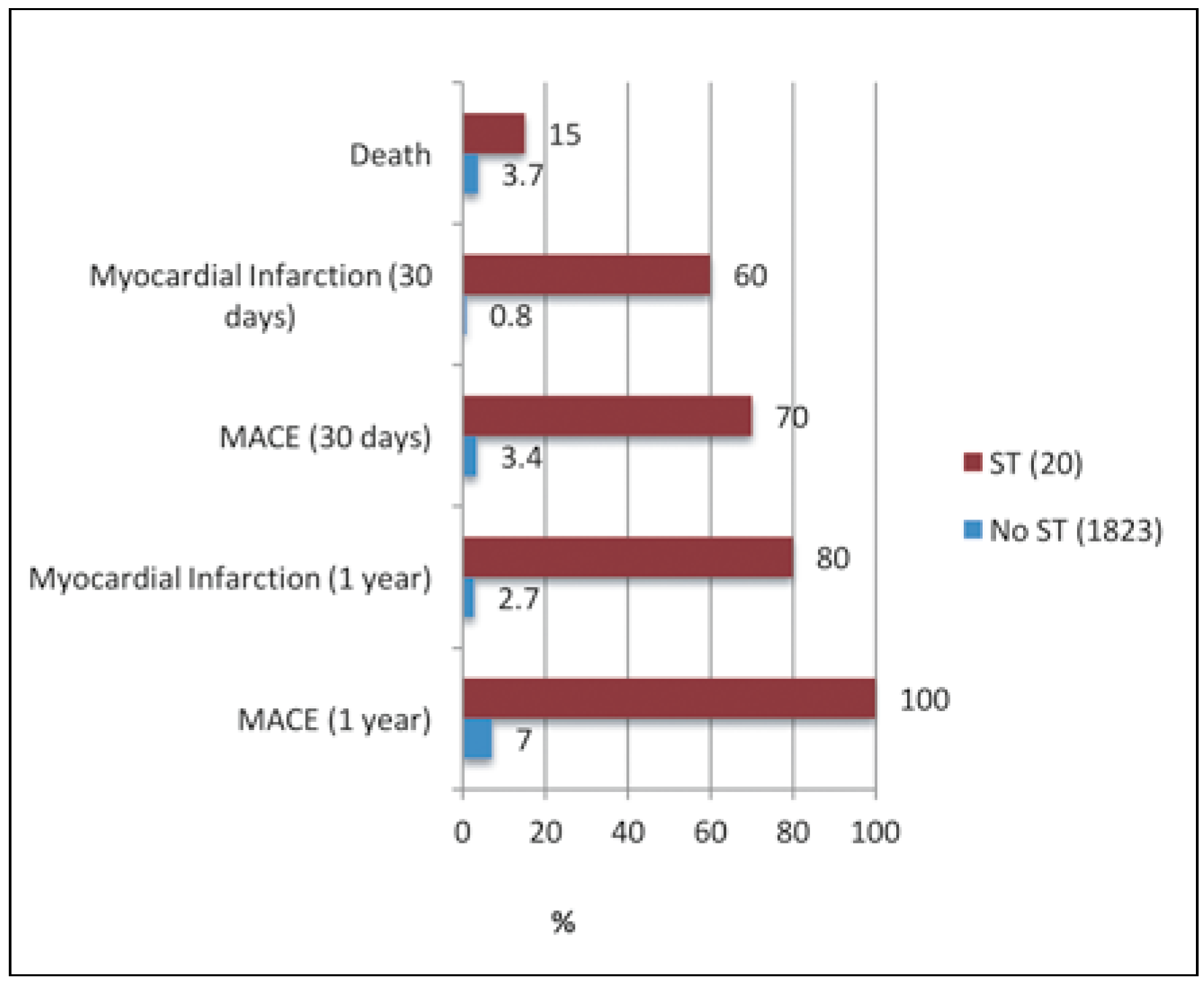
Figure 2.
Outcomes of patient groups with and without stent thrombosis.
Figure 2.
Outcomes of patient groups with and without stent thrombosis.
The (at first glance) surprising finding of a higher stent thrombosis risk in women has already been reported by others [22]. It is probably attributable to the smaller coronary arteries in women; stent diameter is known to be inversely associated with the risk of stent thrombosis [23].
Nonadherence to DAPT has been repeatedly found to be one of the most important risk factors for stent thrombosis, especially in the first 30 days after PCI [8]. All our patients with stent thrombosis started DAPT upon admission, if they were not already on DAPT. Unfortunately, compliance with antiplatelet therapy lacks a useful biomarker and is therefore difficult to assess objectively. In the current cohort it was not investigated in detail and therefor may be one of the factors involved in early stent thrombosis. Data on medication compliance in this study population at 1-year follow up after PCI has been published previously [17], with 96.7% of patients still on aspirin and 81.9% on P2Y12 inhibitors at follow-up (at discharge 99.3 and 100%, respectively). Most frequently, the medication was stopped by the patients’ physicians.
Surprisingly, in our study patients already on clopidogrel prior to primary PCI had a higher risk of stent thrombosis. A possible explanation might be that these patients might generally have been considered at high ischaemic risk by their treating physicians and therefore were left on this P2Y12 inhibitor for prolonged periods of time. As previous reports have mentioned, there may be a benefit of an additional loading dose of prasugrel in patients pretreated with clopidogrel [24].
Premature cessation of DAPT is known as one of the strongest risk factors for early stent thrombosis, but the pathophysiology of late thrombosis is complex. Delayed arterial healing, with inflammatory responses in the vessel wall persisting after stent implantation and delayed endothelial coverage of the stent, has been described as an underlying cause [25]. The risk was highest in first-generation DESs (sirolimusor paclitaxel-eluting stents), and was greatly reduced with the introduction of second-generation DESs (everolimusor zotarolimus-eluting stents) [11], as already mentioned in the introduction. Furthermore, neoatherosclerosis may develop within the stent and be responsible for late stent thrombosis [26]. The sample size of our study was, however, too small to allow differentiation between different types of DES.
Previous reports have described diabetes, in particular in patients requiring insulin, and persistent smoking as having a major influence on stent thrombosis risk [27,28]. Neither finding could be reproduced in our population, possibly as patients with diabetes were under a strict dietary regimen and treated with oral antidiabetic drugs or insulin. Furthermore, the number of patients with diabetes was quite low, which reduced statistical power to detect such a relationship. The percentage of smokers was high, but equal in patients with or without stent thrombosis.
Previously reported angiographic risk factors such as bifurcation lesions, stent overlap or lesion complexity according to American Heart Association/American Collece of Cardiology (AHA/ACC) criteria [11,29] did not affect the risk of stent thrombosis in our patients. In contrast, the use of more than two stents, which might be a surrogate marker for overall lesion burden, was associated with an increased risk of stent thrombosis. Furthermore, in-stent restenosis and coronary lesions distal to the stent increased the risk of stent thrombosis. Thus, it appears that during the procedure stenosis distal to the culprit lesion should be treated appropriately to further reduce the risk of stent thrombosis.
Many other intervention-related factors, such as mismatch of stent and vessel size, stent underexpansion or stent length, are well known risk factors for stent thrombosis [30]. The data acquired in our study did not allow a thorough analysis of these important aspects, as the registry was not designed to allow a detailed investigation of technical factors. Imaging-assisted PCI with the use of intravascular ultrasound (IVUS) or optic coherence tomography (OCT) has shown promising results in reducing the rates of stent thrombosis [31]. Neither IVUS nor OCT were, however, routinely used in our study population, which reflects the clinical practice of four major cardiac centres in Switzerland.
The enrolled patients had characteristics similar to those of larger European studies [32,33] in terms of demographics, medical history and cardiovascular risk profile. The percentage of patients with a history of a prior PCI was slightly higher in our study: 17 compared with 13% in the European Heart Study [32] and in FASTMI [33]. This might be related to the affordable and accessible healthcare system open to all patient groups in Switzerland, with a low intervention threshold. Indeed, as published reports show, patients with clinical signs of coronary artery disease or documented ischaemia are more likely to undergo PCI in Switzerland than in some neighbouring countries [34].
Limitations
One of the limitations of this study is that the current secondary analysis was retrospective and not predefined, albeit the data were gathered prospectively. Furthermore, one of the most important risk factors for stent thrombosis, premature discontinuation of DAPT, was not specifically examined as it was not part of the original study protocol. We acknowledge that data from larger registries when published are always behind technical improvements and recent developments in interventional patient care. As a common issue in studies where large amounts of data are collected, information on, for example, patient history or laboratory results was incompletely acquired and/or documented in a couple of cases. Predictors of stent thrombosis were not evaluated by multivariate analysis. Nevertheless, our results are well in line with the mentioned similar-sized registries and meta-analyses.