Case history
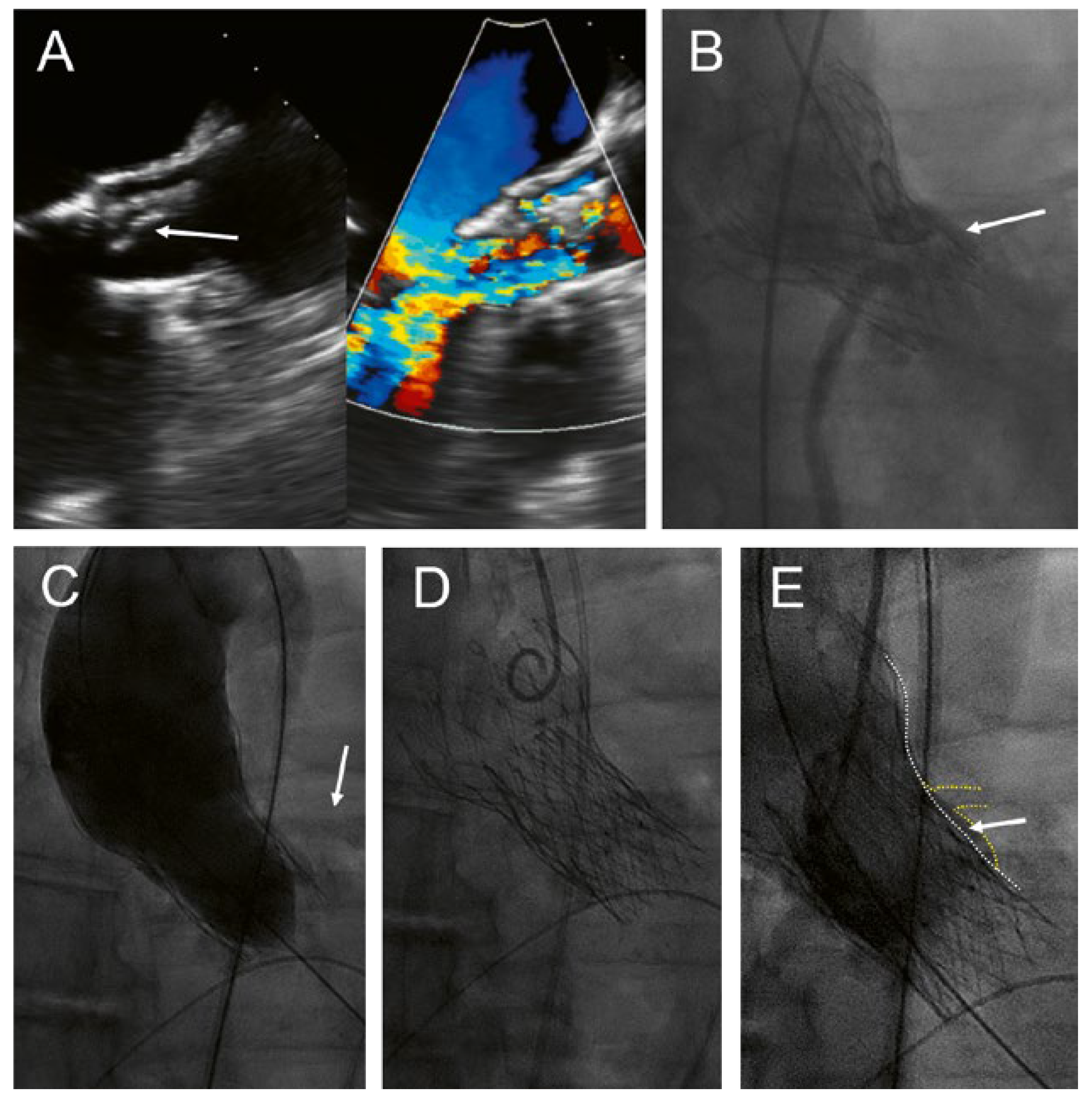
A 68-year-old man presented with increasing shortness of breath and a history of decompensated heart failure 8 years aher transfemoral implantation of a CoreValve 29 mm for severe, calcified aortic stenosis. Transthoracic and transoesophageal echocardiography revealed a partial tear-off and prolapse of one of the bioprosthetic leaflets of the CoreValve, with severe transvalvular and mild paravalvular regurgitation and a mild stenosis with a mean gradient of 26 mm Hg (
Figure 1A). The patient’s main comorbidity was a congenital Laurence-Moon-Bardet-Biedl syndrome with blindness and obesity (body mass index 49 kg/m2). The patient was on rivaroxaban for persistent atrial fibrillation. Coronary angiography showed patent coronary arteries but a very shallow aortic sinus with a substantial risk of coronary obstruction (
Figure 1B). In the presence of negative blood cultures and an unremarkable positron emission tomography (PET) scan, active endocarditis was very unlikely.
The patient was discussed in the interdisciplinary HeartTeam. The decision was to repeat the transcatheter aortic valve implantation with an Allegra transcatheter heart valve (THV) (NVT AG, Muri, Switzerland and NVT GmbH, Hechingen, Germany). Before implantation of the Allegra, a 22-mm TrueDilatation balloon was inflated and a supra-annular injection was performed to ensure patency of the coronary arteries (
Figure 1C). Thereaher, an Allegra 27 mm was implanted about 6 mm below the inflow portion of the CoreValve (
Figure 1D). Postdilatation was performed with the 22-mm TrueDilatation balloon. Postprocedural course was uneventful. Echocardiography before discharge showed a mild paravalvular leak and a mean gradient of 18 mm Hg; the calculated aortic valve area was 1.7 cm2.
Discussion
There is a growing body of evidence for transcatherter aortic valve implantation (TAVI) in degenerated surgical bioprostheses [
1]. However, only a few cases of TAVI in a degenerated TAV have been reported, a condition that we may see frequently in the near future [
2]. Since introduction of the procedure in 2008, the annual numbers of TAVI have rapidly grown in Switzerland, Germany, other countries in Europe and in North America [
3]. Moreover, indications have been extended to younger and lower risk patients. Accordingly, a steady increase of TAVI-in-TAV procedures can be anticipated.
Percutaneous treatment of degenerated THVs offers several challenges. The diameter of the THV usually does not equal the nominal diameter of the THV. Furthermore, as in our case, the degenerated THV may be underexpanded. Therefore, preprocedural screening with multidetector-row computed tomography (MDCT) may be of particular importance. As with to percutaneous treatment of degenerated surgical bioprostheses, there may be a substantial risk for coronary obstruction associated with TAVI-in-TAV procedures. A higher implantation of a second THV may extend the sealing skirt of the first THV. The degenerated leaflets of the first THV, which are effectively stented open by the procedure, may further increase the risk for coronary obstruction. The risk may be highest in patients with a shallow aortic root and a supra-annular THV, as in our patient.
Currently, there is no specific recommendation regarding anticoagulation aher TAVI-in-TAV. Our patient was kept on rivaroxaban, which he was taking because of persistent atrial fibrillation. However, many centres now routinely treat all their patients with a vitamin K antagonist or a direct oral anticoagulant for a few months aher TAVI-in-TAV or TAV in a degenerated surgical bioprosthesis.
All of the currently available THVs can be implanted in degenerated THVs, but they all have some limitations. The Allegra has a relatively straight shape, which minimises interference with the frame of the degenerated THV (
Figure 2) [
4]. In addition, the supra-annular position of the leaflets may result in acceptable gradients even in narrow anatomies or underexpanded THVs. In our patient, other valves may have been less well suited. For example, the height of a Sapien 3 (Edwards Lifesciences, Irvine, USA) may not be sufficient to fully stent a degenerated CoreValve open, especially in the presence of a torn leaflet. Implantation of an Evolut R inside a CoreValve may result in a lot of Nitinol struts inside the aortic sinus and the ascending aorta, resulting in difficult coronary access. Finally, the upper crown of the ACURATE neo (Boston Scientific, Marlborough, USA) may interfere with the frame of the CoreValve, resulting in incomplete expansion. Nevertheless, it may be challenging to access the coronary arteries aher implantation of an Allegra inside a self-expanding valve. In conclusion, this very early experience suggests that the Allegra THV may be well suited for the treatment of degenerated THVs.