Introduction
Orthotopic heart transplantation (HTX) represents the gold standard treatment for patients with advanced heart failure [
1]. The post-transplant survival of adult HTX recipients has consistently improved in past decades: more than 19000 patients have now survived for 20 years and about 100 patients have lived for 25 years following the HTX [
1]. However, HTX has partially lost its positive impact seen during the 1980s and 1990s, probably because of disillusion and disappointment in the increasingly unfavourable imbalance between donors and recipients [
2]. As a result of increasing donorrecipient mismatch, as well as important technical and surgical advances, the attention of the medical community has shihed to ventricular assist devices (VADs) and to the total artificial heart (TAH). Despite great improvements in the devices, so far there has been only modest clinical success in terms of long-term survival and quality of life (QoL) in comparison with HTX [
3]. However, it is important to remember that patients should be referred for possible VAD implantation, since VADs have emerged as the gold standard for bridge-to-transplant in select subjects, as well as a destination therapy when HTX is contraindicated [
3,
4]. Various TAHs have been developed with moderate clinical success, but large size and limited durability presently affect both prognosis and patients’ quality of life [
5].
Case Report
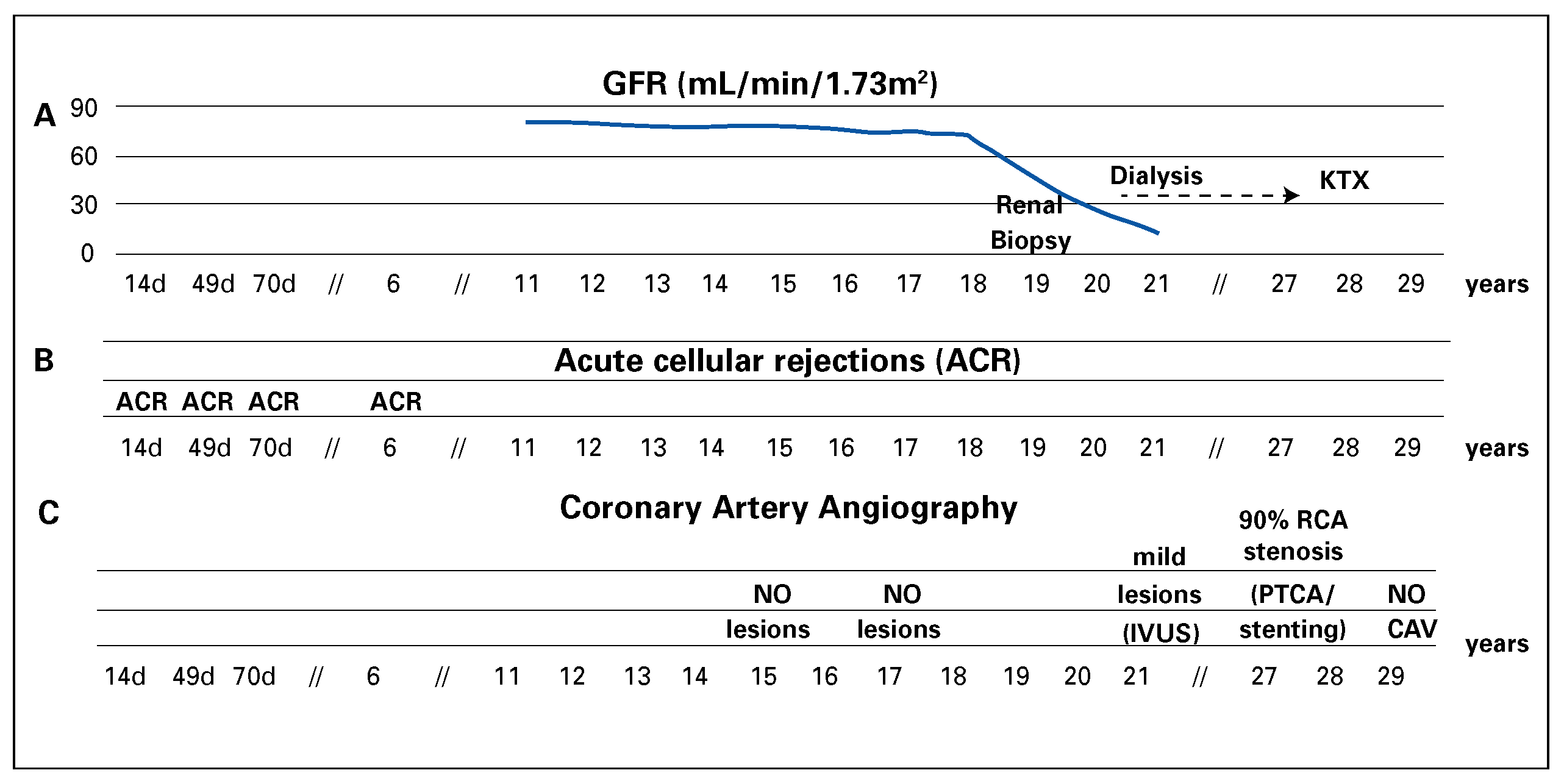
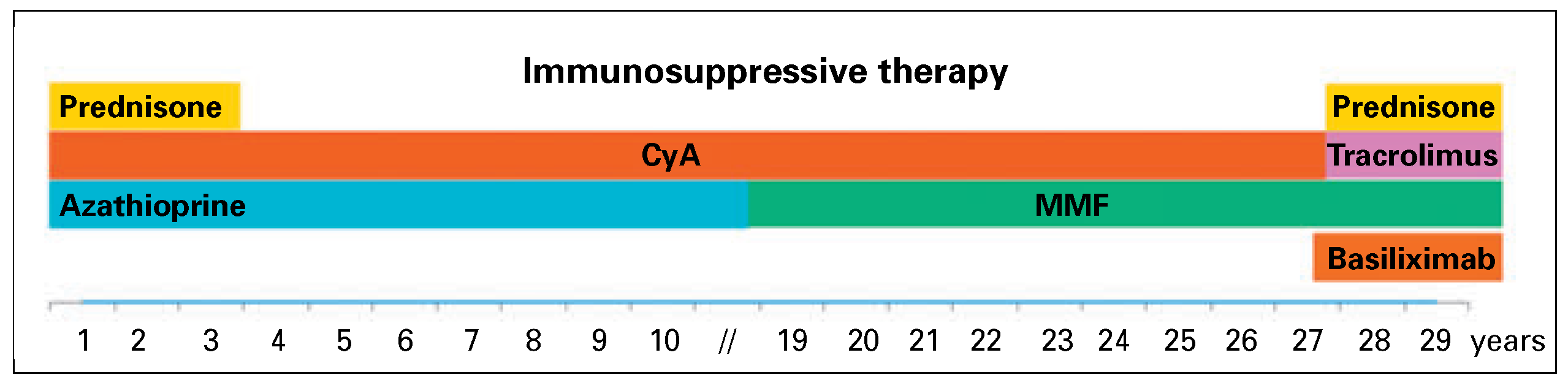
Here we present a case of 29 years of survival aher HTX at age 34 in a Caucasian male, who manifested exceptionally few major post-HTX complications. HTX was performed in 1985 because of advanced heart failure secondary to a dilated cardiomyopathy of unknown aetiology (performed by Prof. M. Turina, Zurich). The patient was otherwise healthy. Mild acute cellular rejections (ACR) occurred 14, 49 and 70 days and 6 years aher HTX. The patient was under an immunosuppression regimen consisting of prednisone (withdrawn after 36 months), ciclosporin and azathioprine, which was replaced by mycophenolate mofetil in 2004 (
Figure 1). Six months aher HTX the patient had complete func-tional recovery and returned to normal everyday activities with good exercise capacity (7 METs by bicycle exercise testing). Follow-up was characterised by good QoL free from further episodes of acute cellular rejection, severe infections or significant malignancies. Multiple cardiovascular risk factors manifested during follow-up (hypertension, type 2 diabetes mellitus and dyslipidaemia) and resulted in lower-limb peripheral artery disease. Surprisingly, serial selective coronary artery angiography revealed only mild focal atherosclerotic coronary stenosis of the right coronary artery (RCA) until 2012, when the patient underwent RCA percutaneous coronary artery intervention with stenting (
Figure 2). Three-vessel coronary intravascular ultrasound performed 20 years aher HTX showed the absence of typical cardiac allograh vasculopathy. During follow-up, the main concern was progressive chronic kidney disease requiring haemodialysis 23 years aher HTX (
Figure 2). The kidney disease was conceivably related to ciclosporin nephrotoxicity and multiple cardiovascular risk factors, as suggested by a renal biopsy performed in 2004.
Subsequently, the patient underwent orthotopic kidney transplantation (KTX) in December 2013 and started a four-drug immunosuppressive regimen including prednisone, mycophenolate mofetil, tacrolimus and basiliximab (
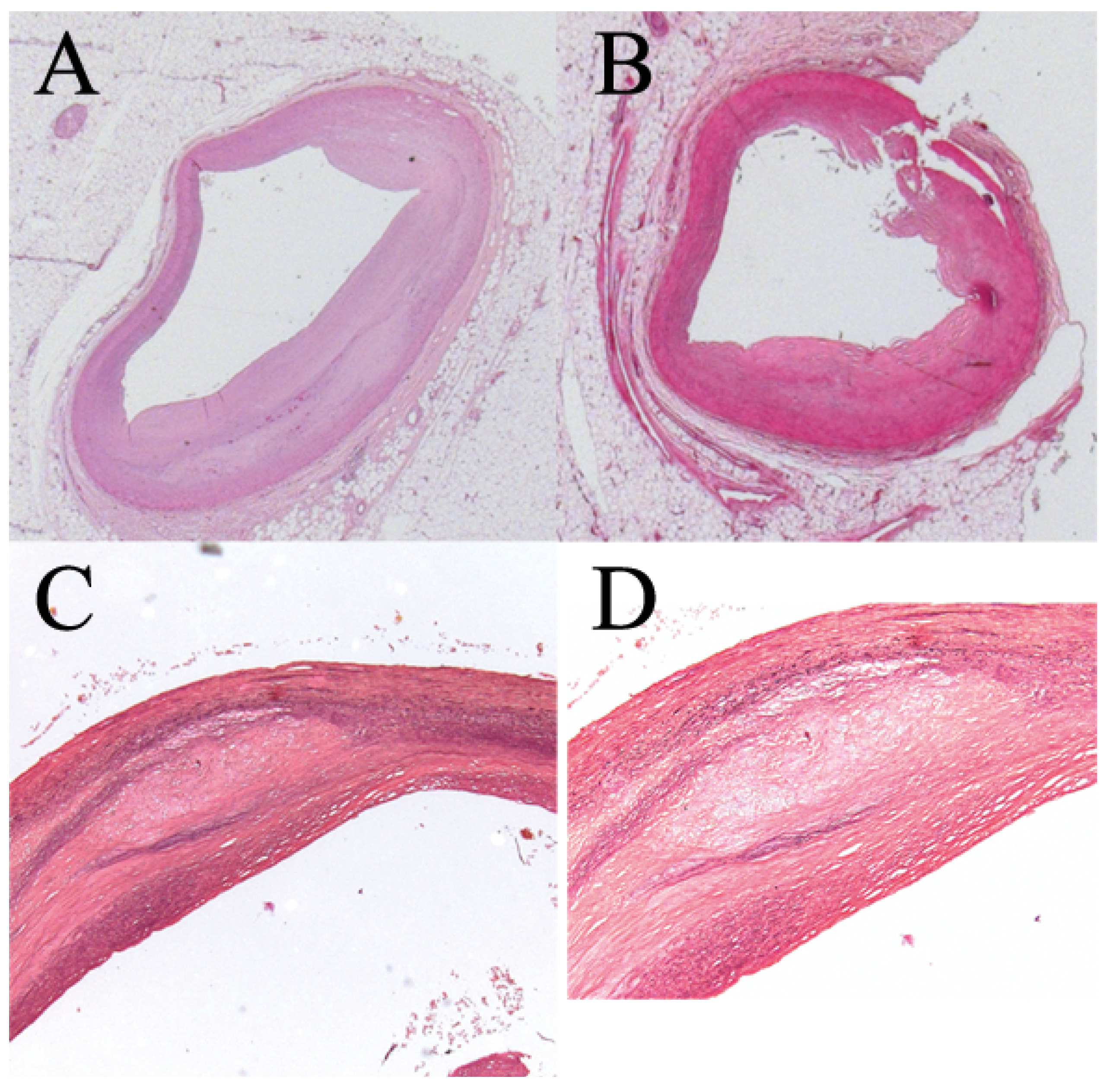
Figure 1). The patient experienced post-transplant cytomegalovirus (CMV) pneumonia (CMV serology R+/D-) resulting in acute respiratory distress syndrome. This was complicated by a Torsades de pointes degenerating into ventricular fibrillation and cardiocirculatory arrest without return of spontaneous circulation. The patient died 3 months aher KTX and 29 years aher HTX. Autopsy confirmed absence of either acute or chronic myocardial rejection and disclosed coronary atherosclerosis without typical cardiac allograh vasculopathy features (
Figure 3).
Since one of the possible explanations for the absence of cardiac allograh vasculopathy might be a favourable immunological matching between donor and recipient and absence of donor specific antibodies, we performed the following analyses: two-digit HLA typing by means of PCR with sequence-specific primers on DNA from the recipient’s and the kidney donor’s peripheral blood cells; serological HLA typing only for the heart donor. HLA mismatches between the cardiac donor and the recipient were found in four out of six loci (HLA-A1, A2, B18, DR4), whereas mismatches with the renal donor were found in three of eight loci (HLAA1, A68, B5). HLA antibody screening for donor-specific antibodies was performed by use of both complementdependent cytotoxicity and single antigen bead assays (Luminex). No donor-specific antibodies against the kidney donor were found, but it was impossible to definitely exclude donor-specific antibodies against the heart donor, since full HLA typing could not be obtained.
Discussion
Numerous features may have affected such a long and mildly complicated survival: male sex, young age of recipient and donor, HTX for neither ischaemic nor congenital cardiomyopathy in an intermediate-to high-volume centre and absence of cardiac allograh vasculopathy are known to be associated with better long-term survival [
1]. The patient’s survival seemed to be only slightly influenced by immunological matching, since cardiac donor-to-recipient mismatch was found in a considerable number of HLA loci (four out of six tested). As demonstrated by biopsy, detrimental renal function results from both cardiovascular risk factors and the iatrogenic effects of ciclosporin, commonly used for immunosuppression following HTX. Similarly, immunosuppression played important role as the cause of CMV pneumonia, thus contributing to the cause of death.
Post-transplant survival of adult HTX recipients has consistently improved in recent decades across all recipient ages and heart disease categories [
1]. However, further significant improvement in survival has been hindered by infections during the early phase and an increase in malignancy, chronic kidney disease and cardiovascular morbidity, especially cardiac allograh vasculopathy, during mid-term and long-term followup [
1].
Our unusual case reinforces the importance of cardiac transplantation, but it is mandatory to bridge several gaps in evidence.
In the last two decades, an increasing number of older recipients (>60 years old) underwent HTX and then received m-TOR (mechanistic target of rapamycin) or calcineurin inhibitors, which have deleterious effects on lipids and renal function [
1]. Therefore, it is of crucial importance to promote evidence-based management of cardiovascular risk factors before and aher HTX, to investigate and promote cardiovascular rehabilitation, and to improve the knowledge of immune and non-immune factors affecting cardiac allograh vasculopathy [
6].
HTX is currently hampered by the increasing number of people waiting for a transplant and the paucity of organs, as for other solid organ transplantations [
7,
8]. In order to overcome these limitations, the medical community should make efforts to increase awareness of organ donation. Similarly, research should focus on enhanced characterisation of the pre-transplant population, on the safe use of non-beating hearts for transplantation [
9] and on re-transplantation, as well as on understanding how the underlying diagnosis of heart failure leading to HTX may interplay with all of the above-mentioned factors [
1]. The management of cellularand antibody-mediated rejection has considerably improved in the last decades, through implementation of both screening protocols before the HTX and new therapeutic protocols of immunosuppression [
10]. However, despite improvements in immunosuppression both during the acute phase and as maintenance therapy, the incidence of rejection during the first year aher HTX was still higher than 10% in the last decade [
1]. Thus, both the implementation of new immunosuppressive drugs and individualised immunosuppressive treatment are mandatory goals for future research.
The scientific and medical community should undertake long-term, vigorous efforts to support research in transplantation and to promote organ donation among the general population, since HTX remains the gold standard treatment for patients with advanced heart failure.