PCSK9-Hemmung mit Antikörpern: Die Resultate der Phase-III-Studienprogramme
Abstract
Einleitung
Hochrisikopatienten: effektive Senkung des LDL-C besonders wichtig
Therapie von Hochrisikopatienten
PCSK9-Hemmer – neue Strategie zur Senkung von LDL-C
Evolocumab – PROFICIO-Studienprogramm
Statin und Evolocumab als Kombinationstherapie
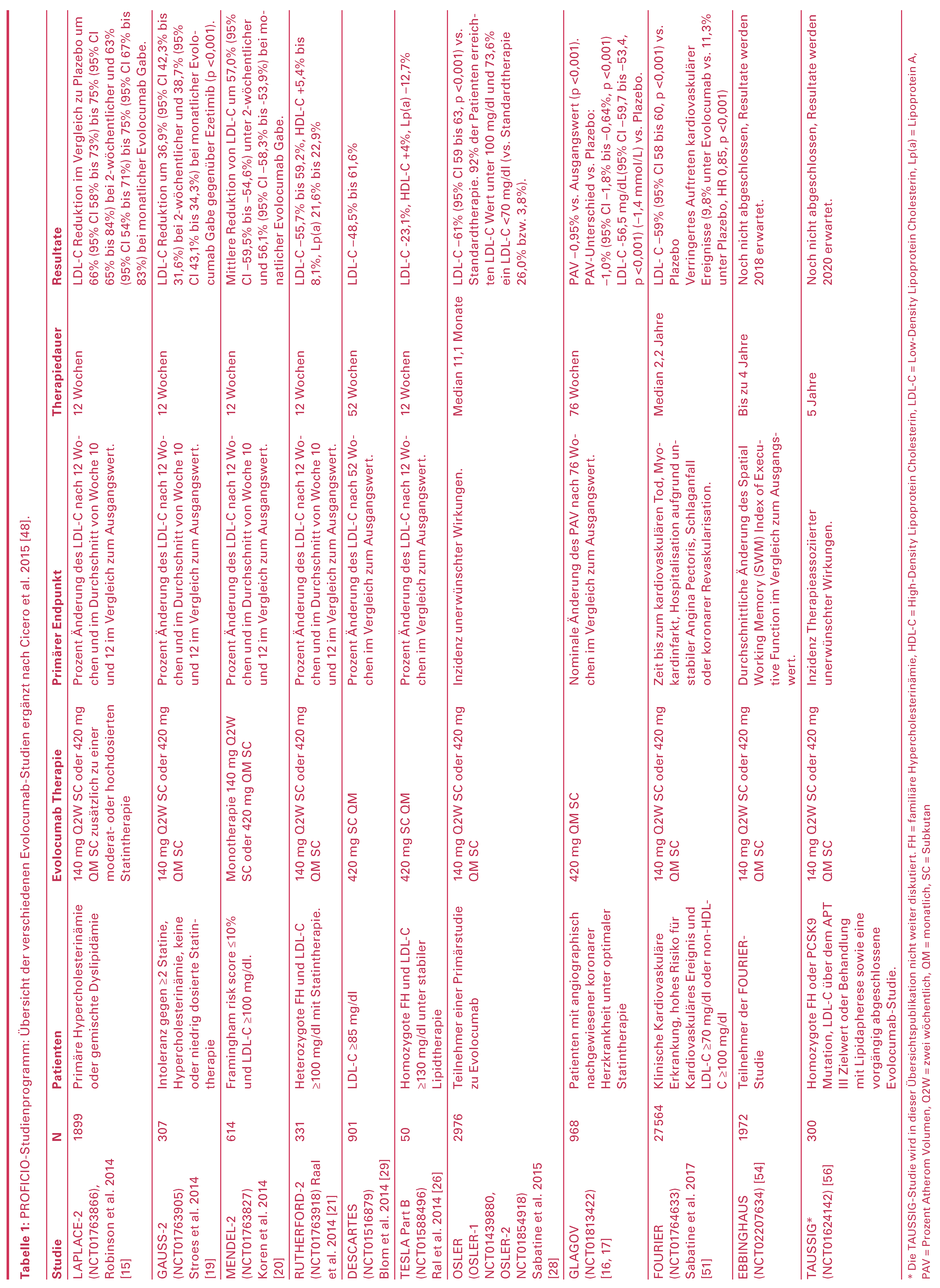 |
Evolocumab bei Statin-Intoleranz
Evolocumab als Monotherapie
Evolocumab bei Patienten mit heterozygoter familiärer Hypercholesterinämie
Evolocumab bei homozygoter familiärer Hypercholesterinämie
Langzeitbehandlung mit Evolocumab
Alirocumab – ODYSSEY-Studienprogramm
Alirocumab in Kombination mit Statinen
Alirocumab bei Statin-Intoleranz
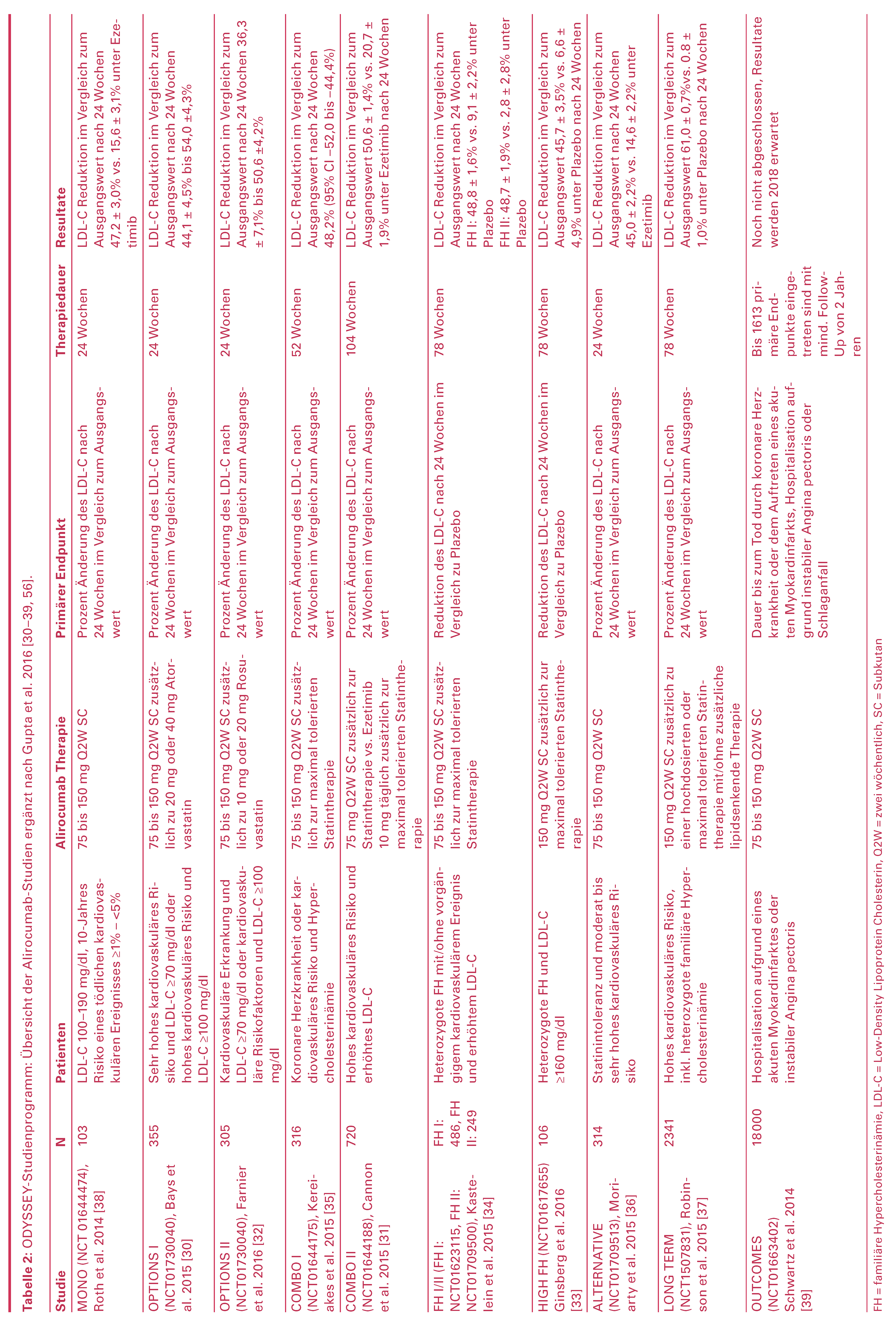 |
Alirocumab als Monotherapie
Alirocumab bei heterozygoter familiärer Hypercholesterinämie
Bococizumab – SPIRE-Studienprogramm
Fazit
Abbreviation
| ApoB | Apolipoprotein B |
| BL | Baseline |
| CI | Konfidenzintervall |
| HDL-C | High-Density Lipoprotein Cholesterin |
| HR | Hazard Ratio |
| LDL-C | Low-Density Lipoprotein Cholesterin |
| LDLR | Low-Density Lipoprotein Rezeptor |
| PAV | Prozentuales Atheromvolumen |
| PBO | Plazebo |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin Type 9 |
| Q2W | 2-wöchentlich |
| QM | Monatlich |
| SC | Subkutan |
| SD | Standardabweichung |
| TAV | Totales Atheromvolumen |
Disclosure Statement
References
- Interpharma. Available online: http://www.interpharma.ch/fakten-statistiken/1815-herz-kreislauf-erkrankungen-als-haeufigste-diagnose.
- Bundesamt für Gesundheit. Nationale Präventionsprogramme – Studie präsentiert erstmalige Berechnungen der direkten und indirekten Kosten der wichtigsten nichtübertragbaren Krankheiten. Bulletin, 14 September.
- Bundesamt für Statistik. Gesundheitsstatistik 2014. Neuchâtel 2014.
- Schweizerische Herzstiftung. Zahlen und Daten über Herz-Kreislauf-Krankheiten in der Schweiz.Bern 2012.
- Miserez, A.R.; Keller, U. Prävention der koronaren Herzkrankheit bei familiären Hypercholesterinämien. Ther. Umsch. 1994, 51, 671–676. [Google Scholar] [PubMed]
- Miserez, A.R.; et al. High prevalence of familial defective apolipoprotein B-100 in Switzerland. J. Lipid Res. 1994, 35, 574–583. [Google Scholar] [CrossRef]
- Miserez, A.R.; et al. Polymorphic haplotypes and recombination rates at the LDL receptor gene locus in subjects with and without familial hypercholesterolemia who are from different populations. Am. J. Hum. Genet. 1993, 52, 808–826. [Google Scholar]
- Goldberg, A.C.; et al. Familial Hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [PubMed]
- Pearson, T.A.; et al. The lipid treatment assessment project (l-tap): A multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch. Intern. Med. 2000, 160, 459–467. [Google Scholar]
- Mancini, G.B.J.; et al. Diagnosis, Prevention, and Management of Statin Adverse Effects and Intolerance: Canadian Working Group Consensus Update. Can. J. Cardiol. 2011, 29, 1553–1568. [Google Scholar] [CrossRef]
- Baigent, C.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010, 376, 1670–1681. [Google Scholar]
- Cannon, C.P.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Norata, G.D.; Ballantyne, C.M.; Catapano, A.L. New therapeutic principles in dyslipidaemia: focus on LDL and Lp(a) lowering drugs. Eur. Heart J. 2013, 34, 1783–1789. [Google Scholar] [CrossRef]
- Mullard, A. Cholesterol-lowering blockbuster candidates speed into Phase III trials. Nat. Rev. Drug Discov. 2012, 11, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; et al. Effect of evolocumab or ezetimibe added to moderateor high-intensity statin therapy on ldl-c lowering in patients with hypercholesterolemia: The laplace-2 randomized clinical trial. JAMA. 2014, 311, 1870–1883. [Google Scholar] [CrossRef]
- Nicholls, S.J.; et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The glagov randomized clinical trial. JAMA. 2016, 316, 2372–2384. [Google Scholar] [CrossRef]
- Puri, R.; et al. Impact of PCSK9 inhibition on coronary atheroma progression: Rationale and design of Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV). Am Heart J. 2016, 176, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; et al. Discontinuation of Statins in Routine Care SettingsA Cohort Study. Ann. Intern. Med. 2013, 158, 526–534. [Google Scholar] [CrossRef]
- Stroes, E.; et al. Anti-PCSK9 Antibody Effectively Lowers Cholesterol in Patients With Statin Intolerance: The GAUSS-2 Randomized, Placebo-Controlled Phase 3 Clinical Trial of Evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Koren, M.J.; et al. Anti-PCSK9 Monotherapy for Hypercholesterolemia: The MENDEL-2 Randomized, Controlled Phase III Clinical Trial of Evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2531–2540. [Google Scholar] [PubMed]
- Raal, F.J.; et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. The Lancet. 2014, 385, 331–340. [Google Scholar] [CrossRef]
- Marais, A.D.; et al. A dose-titration and comparative study of rosuvastatin and atorvastatin in patients with homozygous familial hypercholesterolaemia. Atherosclerosis. 2008, 197, 400–406. [Google Scholar] [CrossRef]
- Raal, F.J.; et al. Expanded-dose simvastatin is effective in homozygous familial hypercholesterolaemia. Atherosclerosis. 1997, 135, 249–256. [Google Scholar] [CrossRef]
- Gagn#233, C.; et al. Efficacy and Safety of Ezetimibe Coadministered With Atorvastatin or Simvastatin in Patients With Homozygous Familial Hypercholesterolemia. Circulation 2002, 105, 2469–2475. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490a. [Google Scholar] [CrossRef]
- Raal, F.J.; et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. The Lancet. 2014, 385, 341–350. [Google Scholar] [CrossRef]
- Pedro-Botet, J.; et al. Apolipoprotein E genotype affects plasma lipid response to atorvastatin in a gender specific manner. Atherosclerosis. 2001, 158, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef]
- Blom, D.J.; et al. A 52-Week Placebo-Controlled Trial of Evolocumab in Hyperlipidemia. N. Engl. J. Med. 2014, 370, 1809–1819. [Google Scholar] [CrossRef]
- Bays, H.; et al. Alirocumab as Add-On to Atorvastatin Versus Other Lipid Treatment Strategies: ODYSSEY OPTIONS I Randomized Trial. J. Clin. Endocrinol. Metab. 2015, 100, 3140–3148. [Google Scholar] [CrossRef]
- Cannon, C.P.; et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur. Heart J. 2015, 36, 1186–1194. [Google Scholar] [CrossRef]
- Farnier, M.; et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: The ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016, 244, 138–146. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; et al. Efficacy and Safety of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia and LDL-C of 160 mg/dl or Higher. Cardiovasc. Drugs Ther. 2016, 30, 473–483. [Google Scholar] [PubMed]
- Kastelein, J.J.P.; et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur. Heart J. 2015, 36, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am. Heart J. 2015, 169, 906–915.e13. [Google Scholar] [CrossRef]
- Moriarty, P.M.; et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J. Clin. Lipidol. 2015, 9, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.M.; et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: Results of a 24 week, double-blind, randomized Phase 3 trial. Int. J. Cardiol. 2014, 176, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: Rationale and design of the ODYSSEY Outcomes trial. Am. Heart J. 2014, 168, 682–689.e1. [Google Scholar] [CrossRef] [PubMed]
- Dadu, R.T.; Ballantyne, C.M. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol. 2014, 11, 563–575. [Google Scholar]
- Ballantyne, C.M.; et al. Results of Bococizumab, A Monoclonal Antibody Against Proprotein Convertase Subtilisin/Kexin Type 9, from a Randomized, Placebo-Controlled, Dose-Ranging Study in Statin-Treated Subjects With Hypercholesterolemia. Am. J. Cardiol. 2015, 115, 1212–1221. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Randomized Clinical Trial Of Bococizumab (PF-04950615; RN316) In Subjects With Hyperlipidemia Or Mixed Dyslipidemia At Risk Of Cardiovascular Events (SPIRE-HR). Available online: http://ClinicalTrials.gov/show/NCT01968954.
- ClinicalTrials.gov. Randomized Clinical Trial of Bococizumab (PF-04950615; RN316) in Subjects With Hyperlipidemia or Mixed Dyslipidemia at Risk of Cardiovascular Events (SPIRE-LDL). Available online: http://ClinicalTrials.gov/show/NCT01968967.
- ClinicalTrials.gov. A 52 Week Study To Assess The Use Of Bococizumab (PF-04950615; RN316) In Subjects With Heterozygous Familial Hypercholesterolemia (SPIRE-FH). In gov. A 52 Week Study To Assess The Use Of Bococizumab (PF-04950615. Available online: http://ClinicalTrials.gov/show/NCT01968980.
- ClinicalTrials.go. The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1). NCT01975376. Available online: http://ClinicalTrials.gov/show/NCT01975376.
- ClinicalTrials.gov. The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2). Available online: http://ClinicalTrials.gov/show/NCT01975389.
- Pfizer. Press release: Pfizer Discontinues Global Development of Bococizumab, Its Investigational PCSK9 Inhibitor. http://www.pfizer.com/news/press-release/press-release-detail/pfizer_discontinues_global_development_of_bococizumab_its_investigational_pcsk9_inhibitor, 2016.
- Ridker, P.M.; et al. Cardiovascular efficacy and sfety of bococizumab in high-risk patients. N. Engl. J. Med. 2017. [CrossRef]
- Cicero, A.F.; Colletti, A.; Borghi, C. Profile of evolocumab and its potential in the treatment of hyperlipidemia. Drug. Des. Devel. Ther. 2015, 9, 3073–3082. [Google Scholar] [CrossRef]
- Sabatine, M.S.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017. [Google Scholar] [CrossRef]
- Swiger, K.; Martin, S. PCSK9 In hibitors and Neurocognitive Adverse Events: Exploring the FDA Directive and a Proposal for N-of-1 Trials. Drug Safety. 2015, 38, 519–526. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.; et al. Molecular Mechanisms Underlying the Effects of Statins in the Central Nervous System. Int. J. Mol. Sci. 2014, 15, 20607. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Evaluating PCSK9 Binding antiBody Influence oN coGnitive HeAlth in High cardiovascUlar Risk Subjects (EBBINGHAUS). Available online: https://clinicaltrials.gov/ct2/show/NCT02207634.
- Jones, P.H.; et al. Safety of Alirocumab (A PCSK9 Monoclonal Antibody) from 14 Randomized Trials. Am. J. Cardiol. 2016, 118, 1805–1811. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Trial Assessing Long Term USe of PCSK9 Inhibition in Subjects With Genetic LDL Disorders (TAUSSIG). Available online: https://clinicaltrials.gov/ct2/show/NCT01624142.
- Gupta, S. Development of proprotein convertase subtilisin/kexin type 9 inhibitors and the clinical potential of monoclonal antibodies in the management of lipid disorders. Vasc. Health Risk Manag. 2016, 12, 421–433. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Attribution-Non-Commercial-NoDerivatives 4.0.
Share and Cite
Mach, F.; Noll, G.; Riesen, W.F.; Sudano, I. PCSK9-Hemmung mit Antikörpern: Die Resultate der Phase-III-Studienprogramme. Cardiovasc. Med. 2017, 20, 123. https://doi.org/10.4414/cvm.2017.00482
Mach F, Noll G, Riesen WF, Sudano I. PCSK9-Hemmung mit Antikörpern: Die Resultate der Phase-III-Studienprogramme. Cardiovascular Medicine. 2017; 20(5):123. https://doi.org/10.4414/cvm.2017.00482
Chicago/Turabian StyleMach, François, Georg Noll, Walter F. Riesen, and Isabella Sudano. 2017. "PCSK9-Hemmung mit Antikörpern: Die Resultate der Phase-III-Studienprogramme" Cardiovascular Medicine 20, no. 5: 123. https://doi.org/10.4414/cvm.2017.00482
APA StyleMach, F., Noll, G., Riesen, W. F., & Sudano, I. (2017). PCSK9-Hemmung mit Antikörpern: Die Resultate der Phase-III-Studienprogramme. Cardiovascular Medicine, 20(5), 123. https://doi.org/10.4414/cvm.2017.00482




