Cardiac Sarcoidosis with Coeliac Disease
Abstract
Case report
Cardiac sarcoidosis
ECG in sarcoidosis
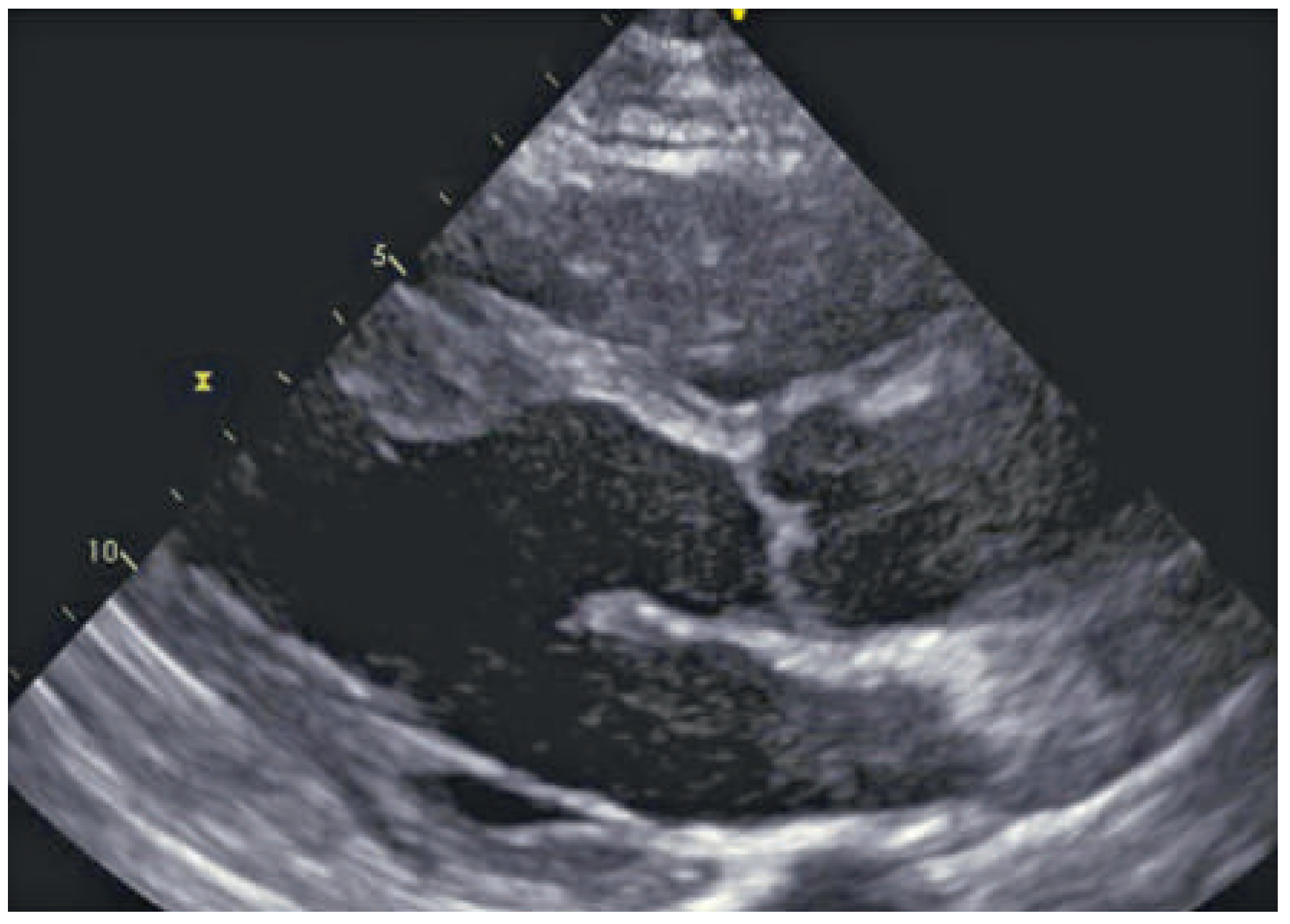
Echocardiography in sarcoidosis
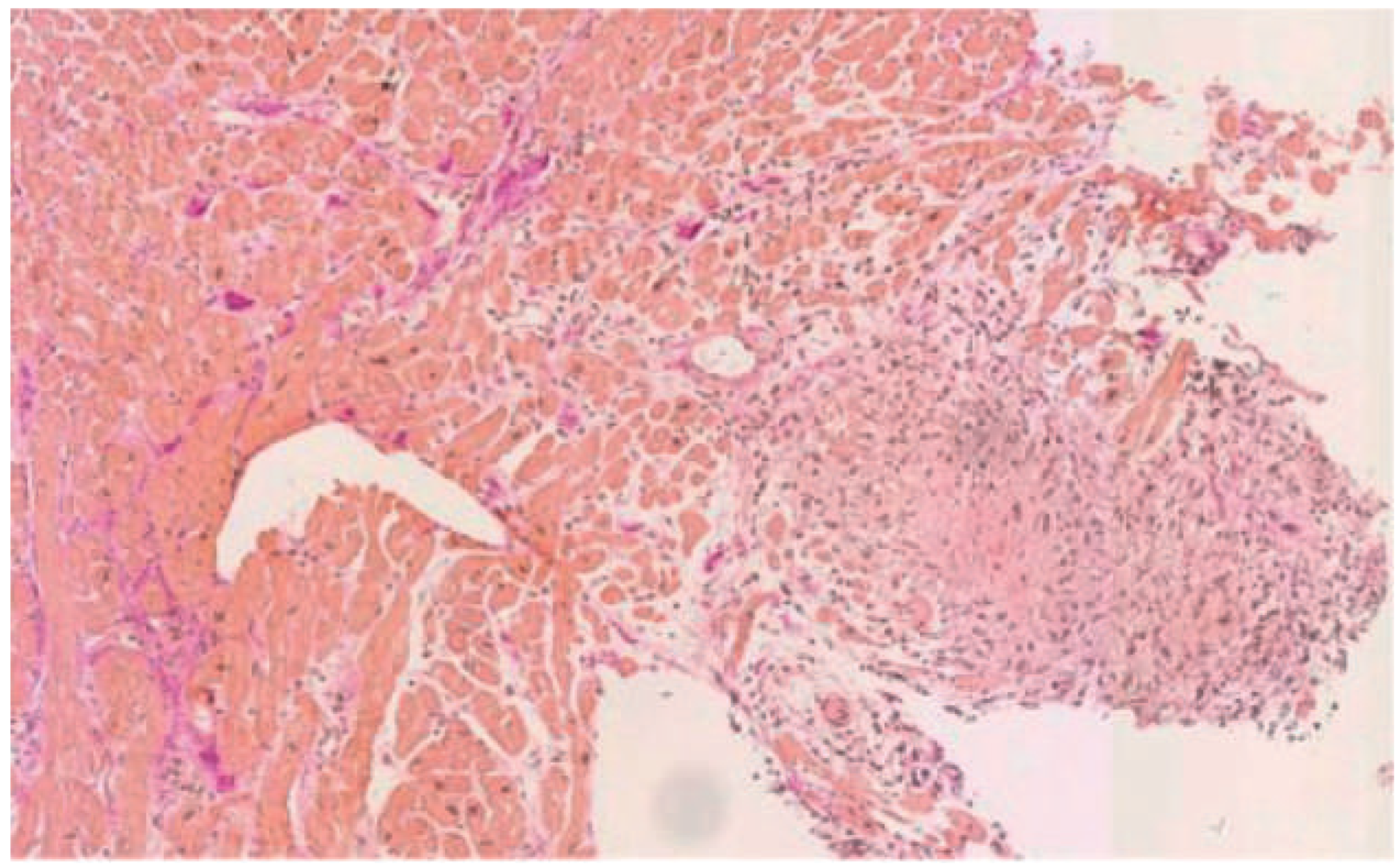
Endomyocardial biopsy in sarcoidosis
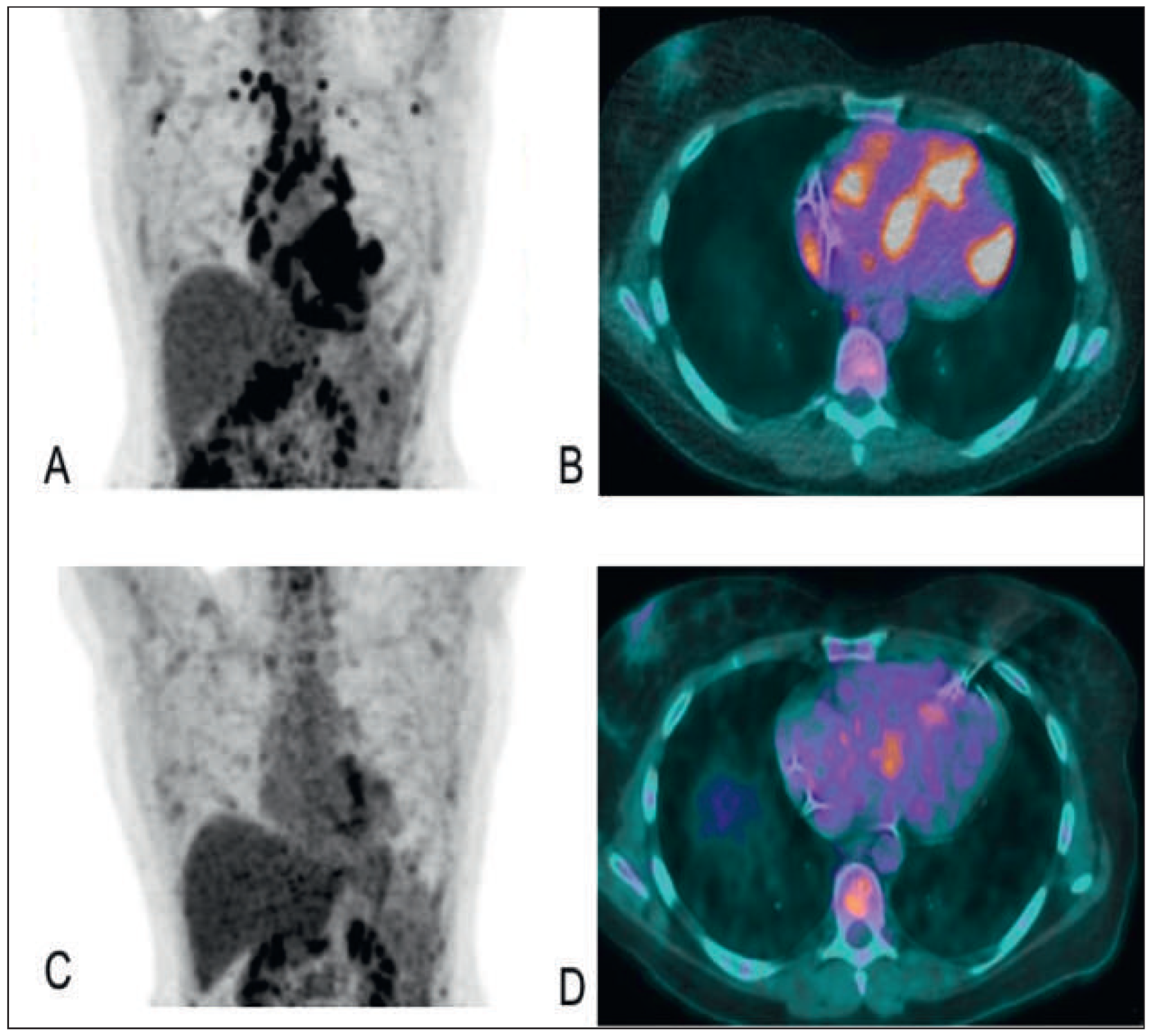
PET in sarcoidosis
Cardiac magnetic resonance imaging
| Cardiac sarcoidosis is confirmed |
| I. Histological diagnosis group: when myocardial biopsy specimens demonstrate noncaseating epithelioid cell granulomas with histological or clinical diagnosis of extracardiac sarcoidosis. |
| II. Clinical diagnosis group: although myocardial biopsy specimens do not demonstrate noncaseating epitheloid cell granulomas, if extracardiac sarcoidosis is diagnosed histologically or clinically and satisfies the following conditions and more than one in six basic diagnostic criteria: |
| II.1. More than two of four major criteria are satisfied: |
| II.1.1. Advanced atrioventricular block. |
| II.1.2. Basal thinning of the interventricular septum. |
| II.1.3. Positive cardiac 67Ga uptake. |
| II.1.4. Depressed LVEF (<50%). |
| II.2. One in four major criteria and more than two in five minor criteria are satisfied: |
| II.2.1. Abnormal ECG findings: ventricular arrhythmias (ventricular tachycardia, multifocal or frequent premature ventricular contractions), complete right bundle-branch block, axis deviation or abnormal Q-wave. |
| II.2.2. Abnormal echocardiography: regional abnormal wall motion or morphological abnormality (ventricular aneurysm, wall thickening). |
| II.2.3. Nuclear medicine: perfusion defect detected by 201Tl myocardial scintigraphy or 99Tc myocardial scintigraphy. |
| II.2.4. Gadolinium-enhanced MRI: delayed enhancement of myocardium. |
| II.2.5. Endomyocardial biopsy: interstitial fibrosis or monocyte infiltration over moderate grade. |
Management
Immunosuppressive therapy
Cardiac pharmacological therapy
Arrhythmias
Implantable cardioverter defibrillator
Radiofrequency ablation
Disclosure statement
References
- Reggoug, S.; Benelbarhdadi, I.; Essamri, W.; Ajana, F.Z.; Afifi, R.; Benazzouz, M.; et al. Celiac disease associated with sarcoidosis. Gastroenterol Clin Biol. 2009, 33, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Perez-Verdia, A.; Angulo, F.; Hardwicke, F.L.; Nugent, K.M. Acute cardiac toxicity associated with high-dose intravenous methotrexate therapy: case report and review of the literature. Pharmacotherapy 2005, 25, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Golwala, H.; Dernaika, T. Atrial fibrillation as the initial clinical manifestation of cardiac sarcoidosis: a case report and review of the literature. J Cardiovasc Med (Hagerstown). 2015, 16 (Suppl. S2), S104–S112. [Google Scholar] [CrossRef] [PubMed]
- Ardehali, H.; Howard, D.L.; Hariri, A.; Qasim, A.; Hare, J.M.; Baughman, K.L.; et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 2005, 150, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disorders. 2007, 27, 89–102.
© 2016 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Fournier, S.; Regamey, J.; Rotman, S.; Pruvot, E.; Hullin, R. Cardiac Sarcoidosis with Coeliac Disease. Cardiovasc. Med. 2016, 19, 128. https://doi.org/10.4414/cvm.2016.00412
Fournier S, Regamey J, Rotman S, Pruvot E, Hullin R. Cardiac Sarcoidosis with Coeliac Disease. Cardiovascular Medicine. 2016; 19(4):128. https://doi.org/10.4414/cvm.2016.00412
Chicago/Turabian StyleFournier, Stéphane, Julien Regamey, Samuel Rotman, Etienne Pruvot, and Roger Hullin. 2016. "Cardiac Sarcoidosis with Coeliac Disease" Cardiovascular Medicine 19, no. 4: 128. https://doi.org/10.4414/cvm.2016.00412
APA StyleFournier, S., Regamey, J., Rotman, S., Pruvot, E., & Hullin, R. (2016). Cardiac Sarcoidosis with Coeliac Disease. Cardiovascular Medicine, 19(4), 128. https://doi.org/10.4414/cvm.2016.00412




