Symptomatic Polymorphic Ventricular Tachycardia in a Young Woman
Abstract
Question
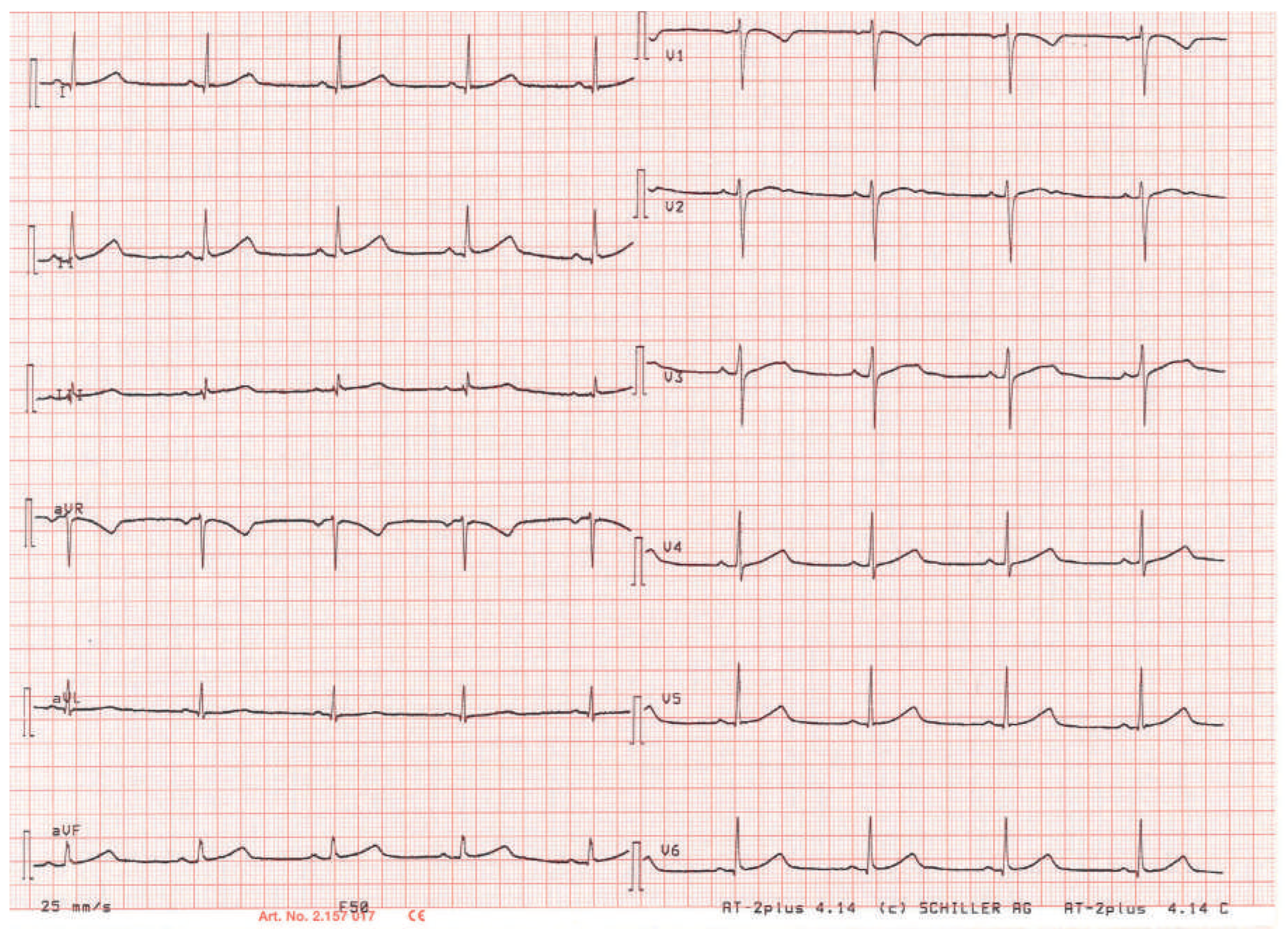
Clinical History
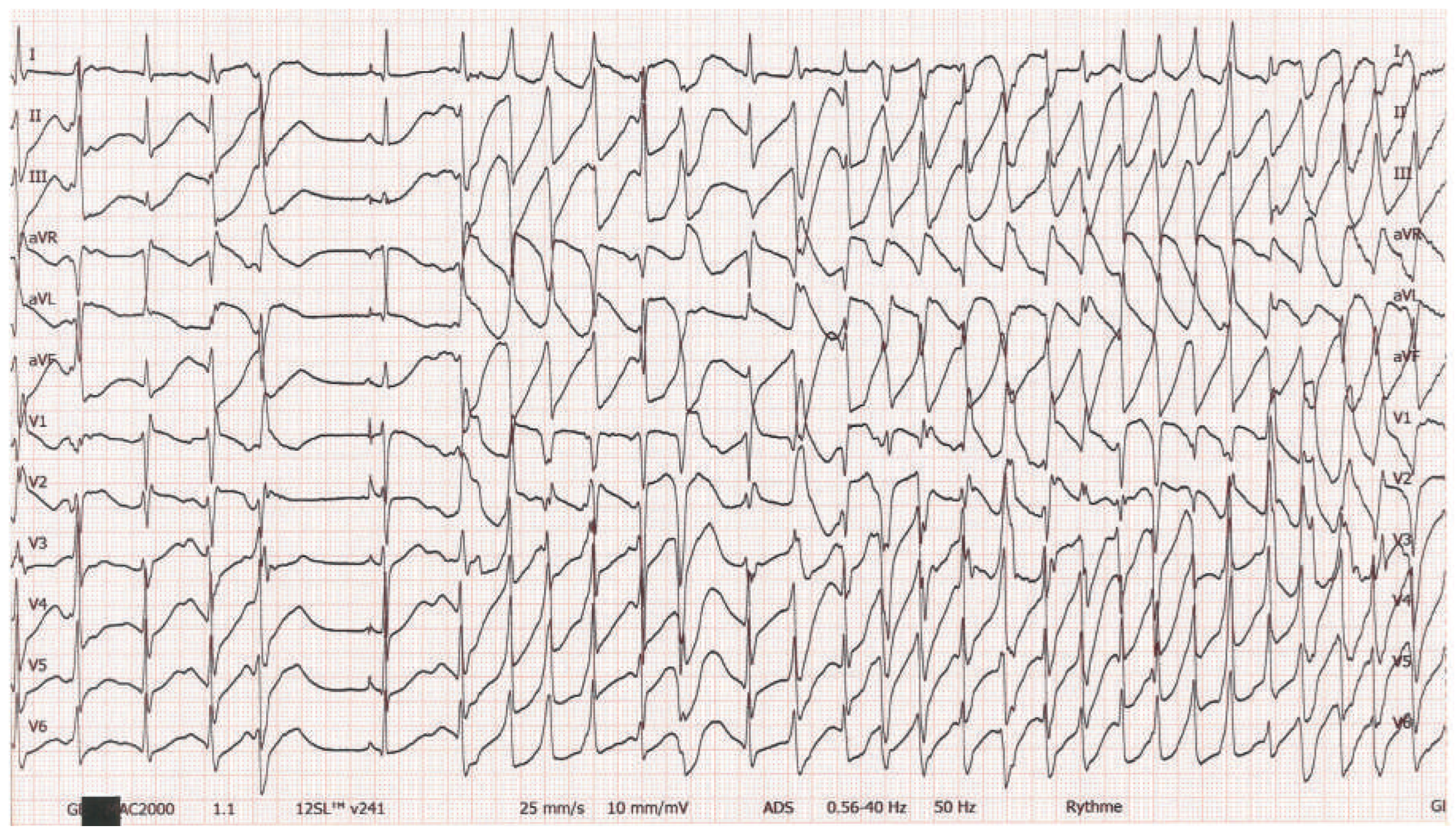
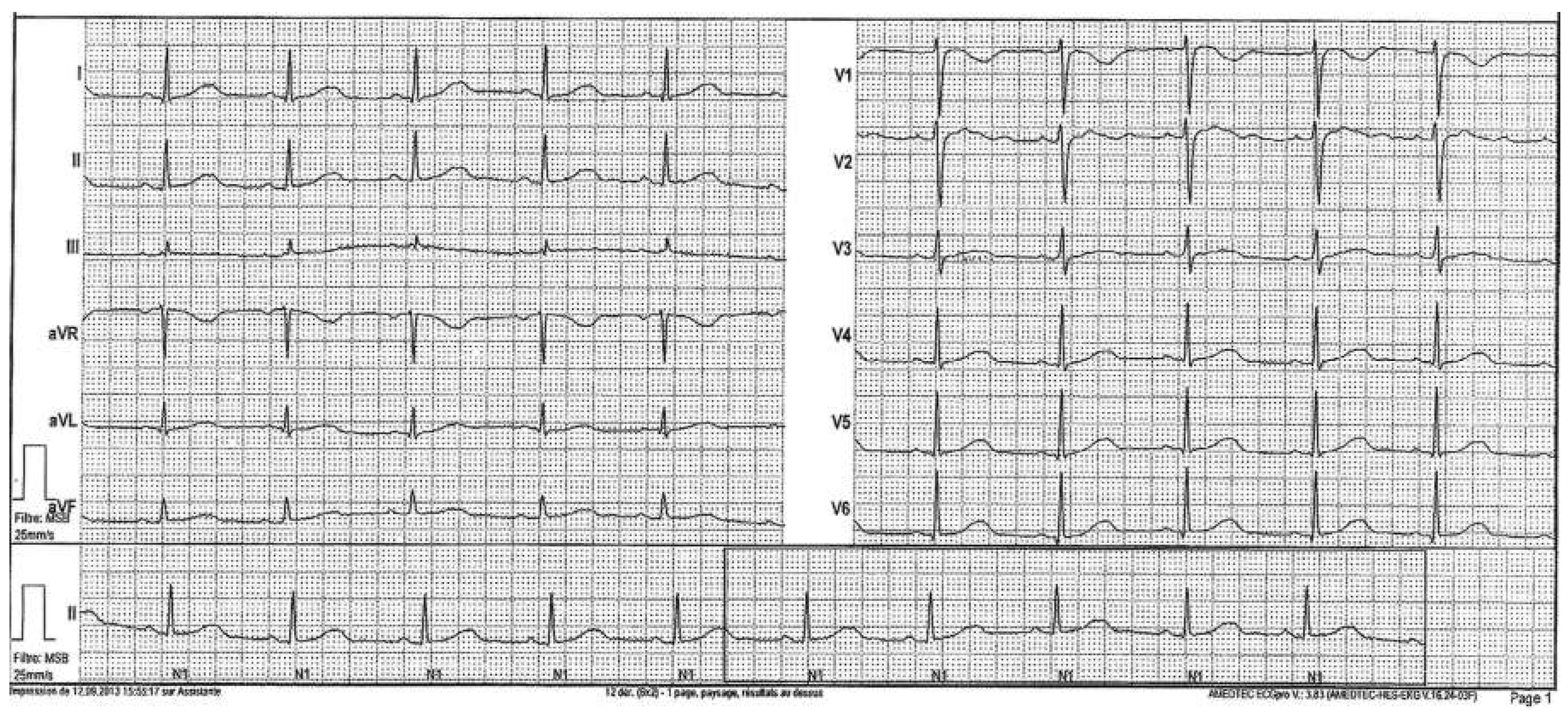
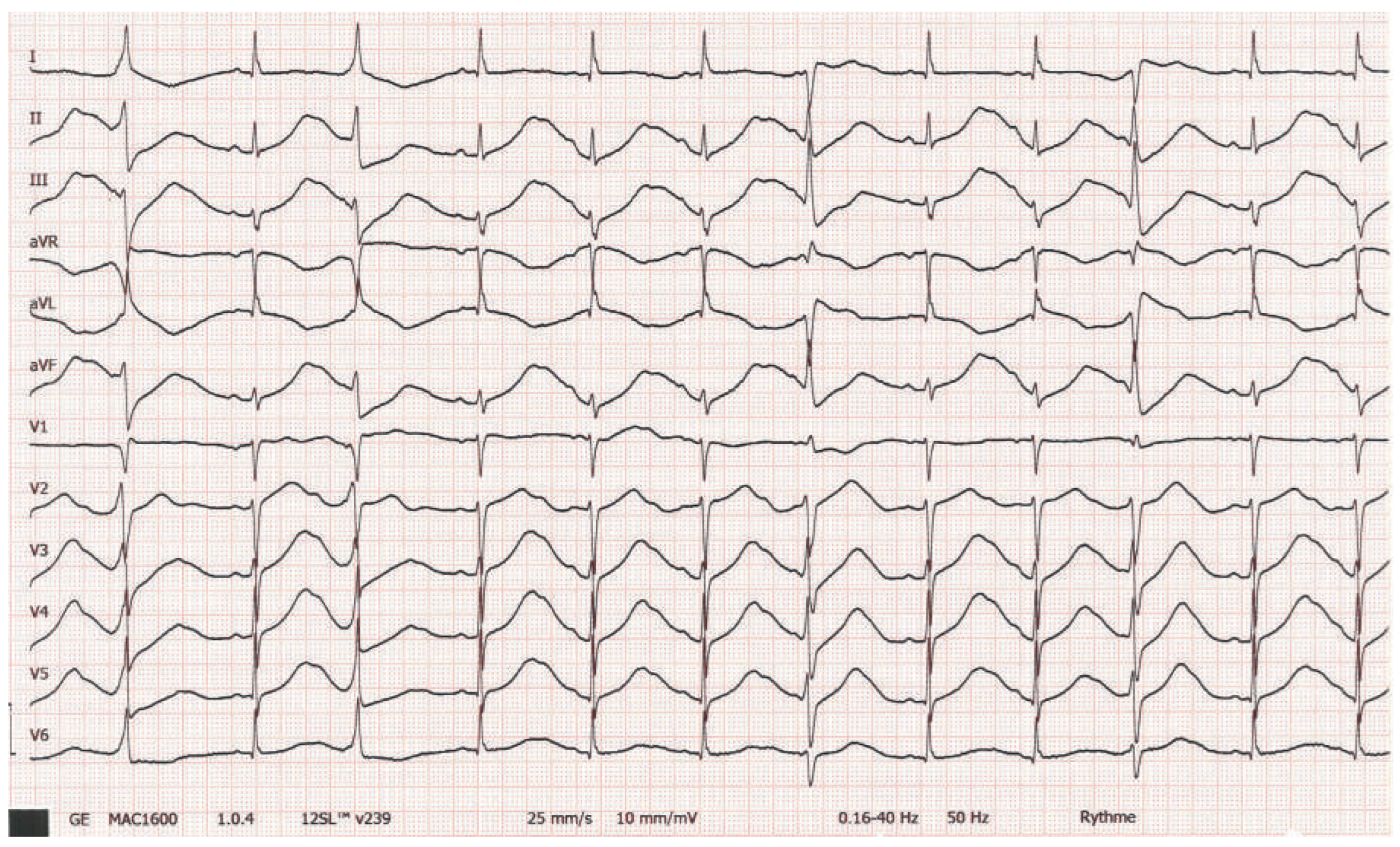
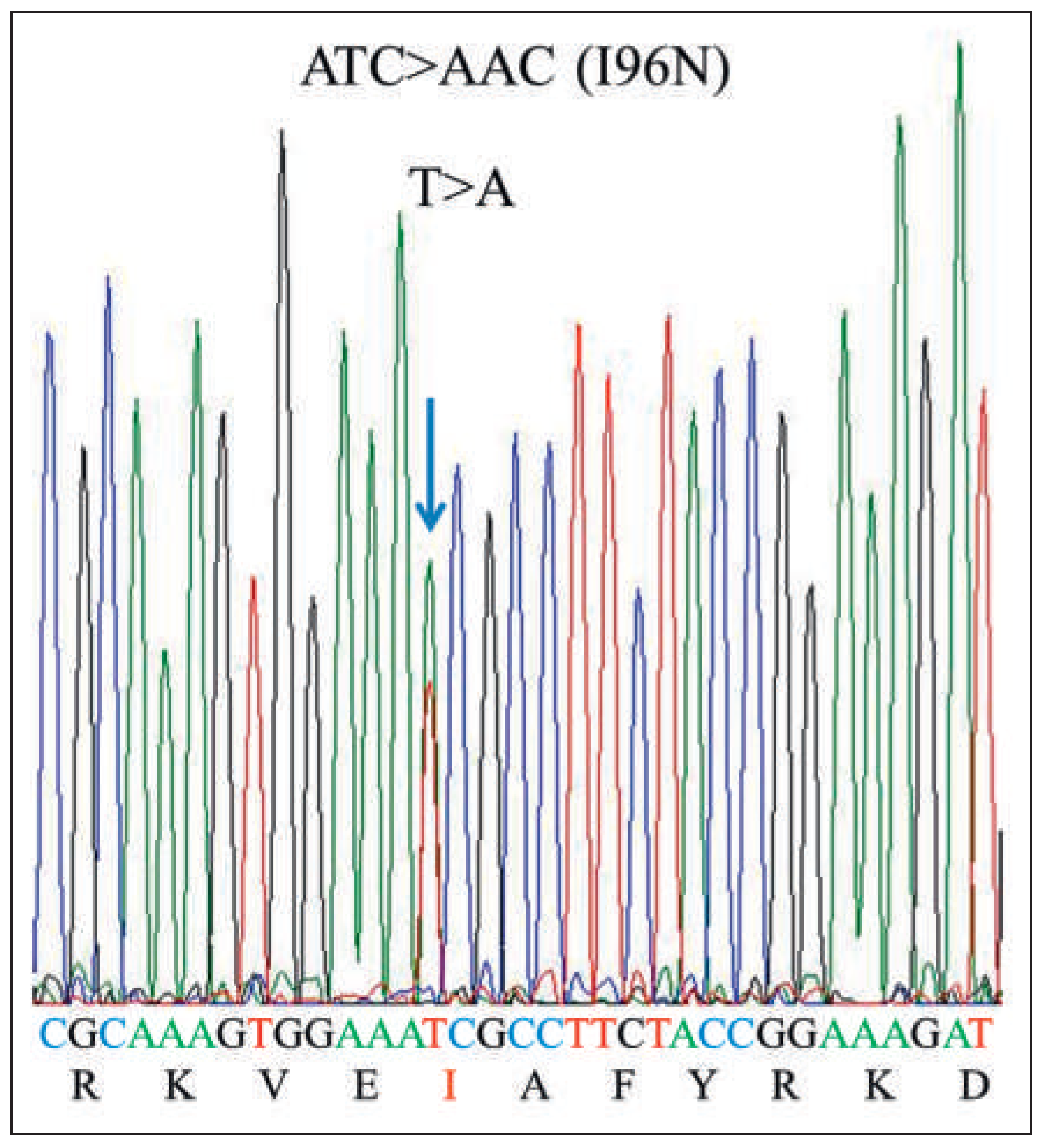
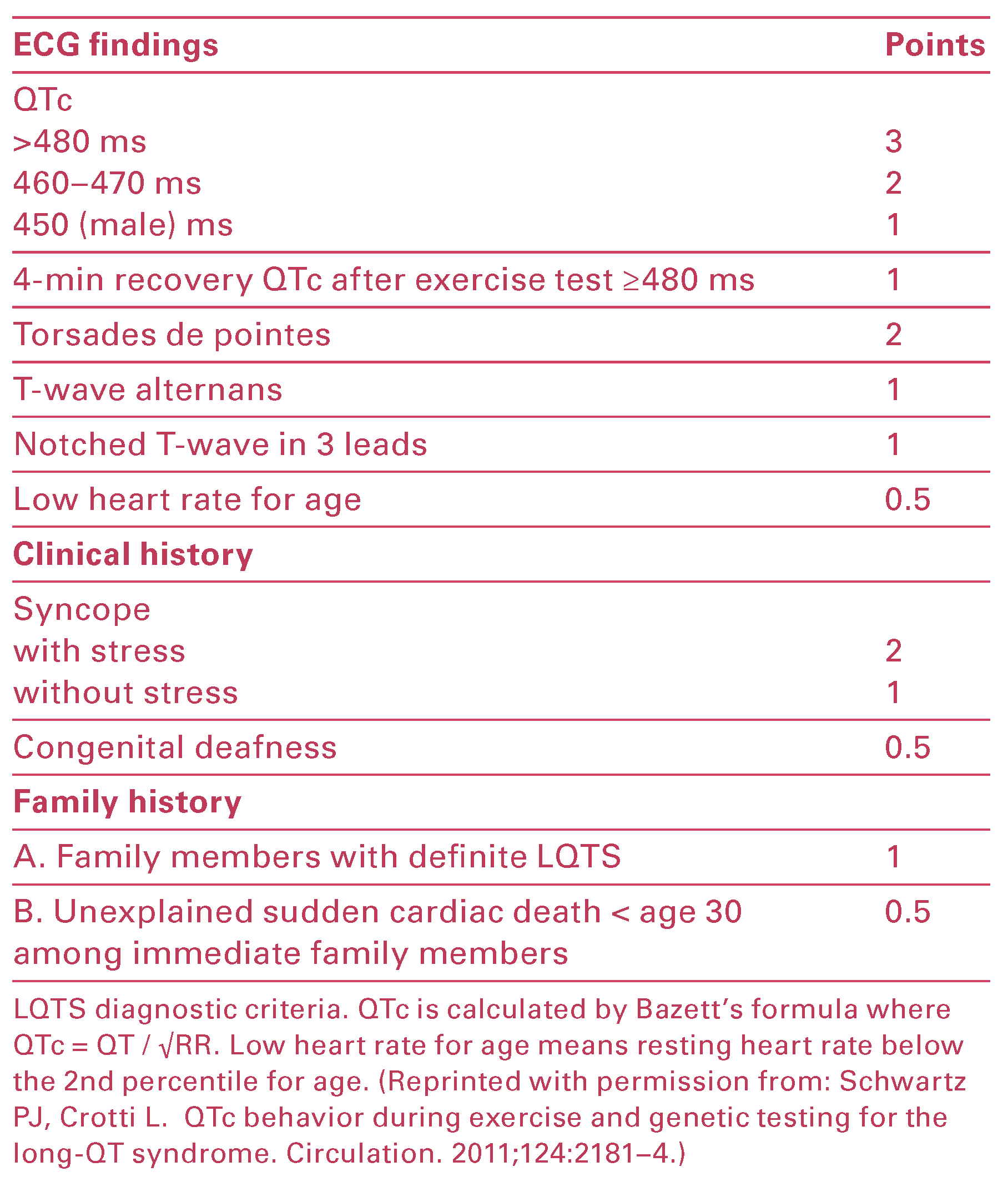
Workup and Evolution
Discussion
Disclosure Statement
References
- Harmon, K.G.; Asif, I.M.; Klossner, D.; Drezner, J.A. Incidence of sudden cardiac death in national collegiate athletic association athletes. Circulation 2011, 123, 1594–600. [Google Scholar] [CrossRef]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009, 119, 1085–92. [Google Scholar] [CrossRef]
- Marijon, E.; Tafflet, M.; Celermajer, D.S.; Dumas, F.; Perier, M.C.; Mustafic, H.; et al. Sports-related sudden death in the general population. Circulation 2011, 124, 672–81. [Google Scholar] [CrossRef]
- Harmon, K.G.; Asif, I.M.; Klossner, D.; Drezner, J.A. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011, 123, 1594–600. [Google Scholar] [PubMed]
- Maron, B.J.; Thompson, P.D.; Ackerman, M.J.; Balady, G.; Berger, S.; Cohen, D.; et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007, 115, 1643–455. [Google Scholar]
- Corrado, D.; Pelliccia, A.; Bjørnstad, H.H.; Vanhees, L.; Biffi, A.; Borjesson, M.; et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2005, 26, 516–524. [Google Scholar]
- Bille, K.; Figueiras, D.; Schamasch, P.; Kappenberger, L.; Brenner, J.I.; Meijboom, F.J.; et al. Sudden cardiac death in athletes: the Lausanne Recommendations. Eur J Cardiovasc Prev Rehabil. 2006, 13, 859–75. [Google Scholar]
- Dvorak, J.; Grimm, K.; Schmied, C.; Junge, A. Development and implementation of a standardized precompetition medical assessment of international elite football players--2006 FIFA World Cup Germany. Clin J Sport Med. 2009, 19, 316–21. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Fagard, R.; Bjørnstad, H.H.; Anastassakis, A.; Arbustini, E.; Assanelli, D.; et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005, 26, 1422–45. [Google Scholar] [PubMed]
- Drezner, J.A.; Rogers, K.J. Sudden cardiac arrest in intercollegiate athletes: detailed analysis and outcomes of resuscitation in nine cases. Heart Rhythm. 2006, 3, 755–759. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Schiavon, M.; Thiene, G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998, 339, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Pavei, A.; Michieli, P.; Schiavon, M.; Thiene, G. Trends in Sudden Cardiovascular Death in Young Competitive Athletes After Implemention of a Preparticipation Screening Program. JAMA 2006, 296, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Corrado, D.; Marcus, F.I.; Nava, A.; Thiene, G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 2009, 373, 1289–300. [Google Scholar] [CrossRef]
- Corrado, D.; Pelliccia, A.; Heidbuchel, H.; Sharma, S.; Link, M.; Basso Cet, a.l. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J 2010, 31, 243–59. [Google Scholar] [CrossRef]
- Baggish, A.L.; Hutter, A.M., Jr.; Wang, F.; Yared, K.; Weiner, R.B.; Kupperman, E.; et al. Cardiovascular screening in college athletes with and without electrocardiography: A cross-sectional study. Ann Intern Med. 2010, 152, 269–75. [Google Scholar] [CrossRef]
- Riding, N.R.; Salah, O.; Sharma, S.; Carré, F.; George, K.P.; Farooq, A.; et al. ECG and morphologic adaptations in Arabic athletes: are the European Society of Cardiology’s recommendations for the interpretation of the 12-lead ECG appropriate for this ethnicity? Br J Sports Med 2014, 48, 1138–43. [Google Scholar] [CrossRef]
- Papadakis, M.; Carre, F.; Kervio, G.; Rawlins, J.; Panoulas, V.F.; Chandra, N.; et al. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur Heart J 2011, 32, 2304–13. [Google Scholar] [CrossRef]
- Magalski, A.; McCoy, M.; Zabel, M.; Magee, L.M.; Goeke, J.; Main, M.L.; et al. Cardiovascular screening with electrocardiography and echocardiography in collegiate athletes. Am J Med 2011, 124, 511–8. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A.; Stein, R.; Perez, M.V.; Freeman, J.; Wheeler, M.; Dewey, F.; et al. Interpretation of the electrocardiogram of young athletes. Circulation 2011, 124, 746–57. [Google Scholar] [CrossRef]
- Drezner, J.A.; Ackerman, M.J.; Anderson, J.; Ashley, E.; Asplund, C.A.; Baggish, A.L.; et al. Electrocardiographic interpretation in athletes: the ‘Seattle Criteria’. Br J Sports Med 2013, 47, 122–124. [Google Scholar] [CrossRef]
- Drezner, J.A.; Fischbach, P.; Froelicher, V.; Marek, J.; Pelliccia, A.; Prutkin, J.M.; et al. Normal electrocardiographic findings: recognising physiological adaptations in athletes. Br J Sports Med 2013, 47, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Drezner, J.A.; Ackerman, M.J.; Cannon, B.C.; Corrado, D.; Heidbuchel, H.; Prutkin, J.M.; et al. Abnormal electrocardiographic findings in athletes: recognising changes suggestive of primary electrical disease. Br J Sports Med 2013, 47, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Drezner, J.A.; Ashley, E.; Baggish, A.L.; Börjesson, M.; Corrado, D.; Owens, D.S.; et al. Abnormal electrocardiographic findings in athletes: recognising changes suggestive of cardiomyopathy. Br J Sports Med 2013, 47, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.; La Gerche, A.; Kalman, J.; Lo, W.; Fallon, K.; MacIsaac, A.; et al. The Seattle Criteria increase the specificity of preparticipation ECG screening among elite athletes. Br J Sports Med 2014, 48, 1144–50. [Google Scholar] [CrossRef]
- Riding, N.R.; Sheikh, N.; Adamuz, C.; Watt, V.; Farooq, A.; Whyte, G.P.; et al. Comparison of three current sets of electrocardiographic interpretation criteria for use in screening athletes. Heart. 2015, 101, 384–90. [Google Scholar] [CrossRef]
- Pickham, D.; Zarafshar, S.; Sani, D.; Kumar, N.; Froelicher, V. Comparison of three ECG criteria for athlete pre-participation screening. J Electrocardiol. 2014, 47, 769–74. [Google Scholar] [CrossRef]
- Sheikh, N.; Papadakis, M.; Ghani, S.; Zaidi, A.; Gati, S.; Adami, P.E. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation. 2014, 129, 1637–49. [Google Scholar] [CrossRef]
© 2016 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
dos Santos Rocha, A.; Bhuyian, Z.A.; Fellman, F.; Schlaepfer, J.; Bovy, M.; Delabays, A. Symptomatic Polymorphic Ventricular Tachycardia in a Young Woman. Cardiovasc. Med. 2016, 19, 90. https://doi.org/10.4414/cvm.2016.00395
dos Santos Rocha A, Bhuyian ZA, Fellman F, Schlaepfer J, Bovy M, Delabays A. Symptomatic Polymorphic Ventricular Tachycardia in a Young Woman. Cardiovascular Medicine. 2016; 19(3):90. https://doi.org/10.4414/cvm.2016.00395
Chicago/Turabian Styledos Santos Rocha, André, Zahurul Alam Bhuyian, Florence Fellman, Jürg Schlaepfer, Michèle Bovy, and Alain Delabays. 2016. "Symptomatic Polymorphic Ventricular Tachycardia in a Young Woman" Cardiovascular Medicine 19, no. 3: 90. https://doi.org/10.4414/cvm.2016.00395
APA Styledos Santos Rocha, A., Bhuyian, Z. A., Fellman, F., Schlaepfer, J., Bovy, M., & Delabays, A. (2016). Symptomatic Polymorphic Ventricular Tachycardia in a Young Woman. Cardiovascular Medicine, 19(3), 90. https://doi.org/10.4414/cvm.2016.00395




