The Impact of Fractional Flow Reserve and iFr on Current PCI Strategies
Abstract
Introduction
What is Fractional Flow Reserve?
Importance of Ischaemia in Stable Coronary Artery Disease Management
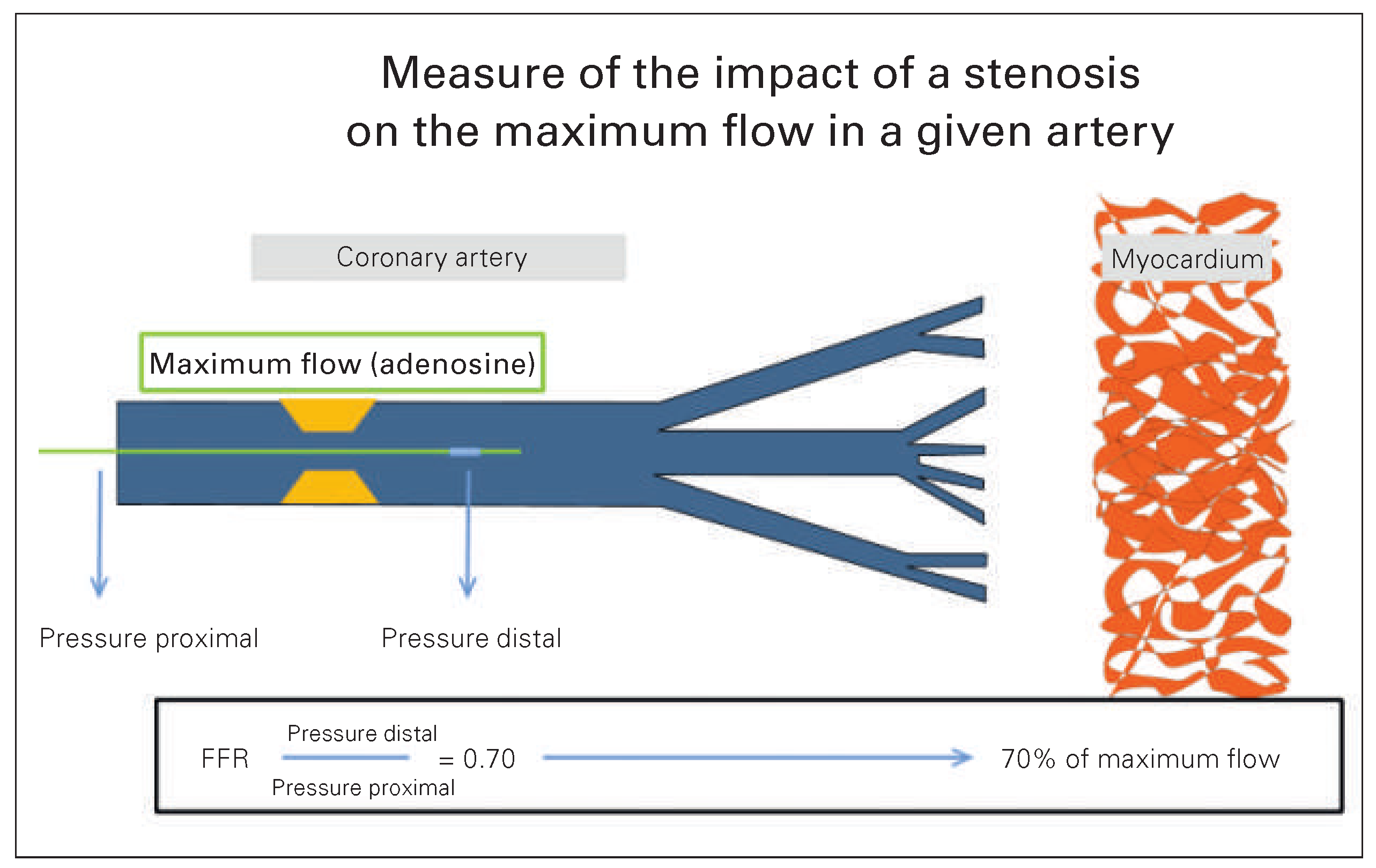
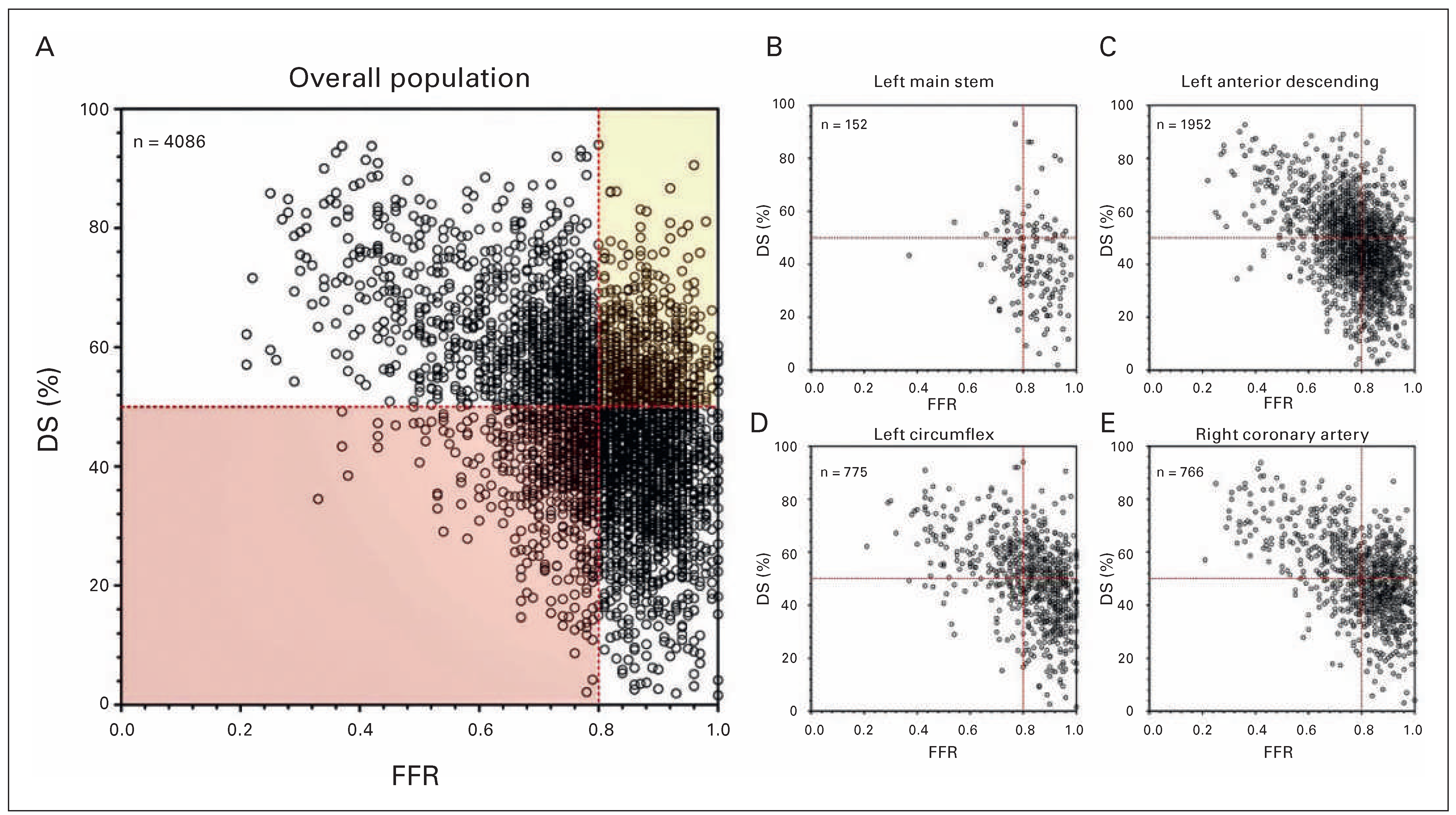
The Value of Coronary Angiography in Detecting Myocardial Ischaemia
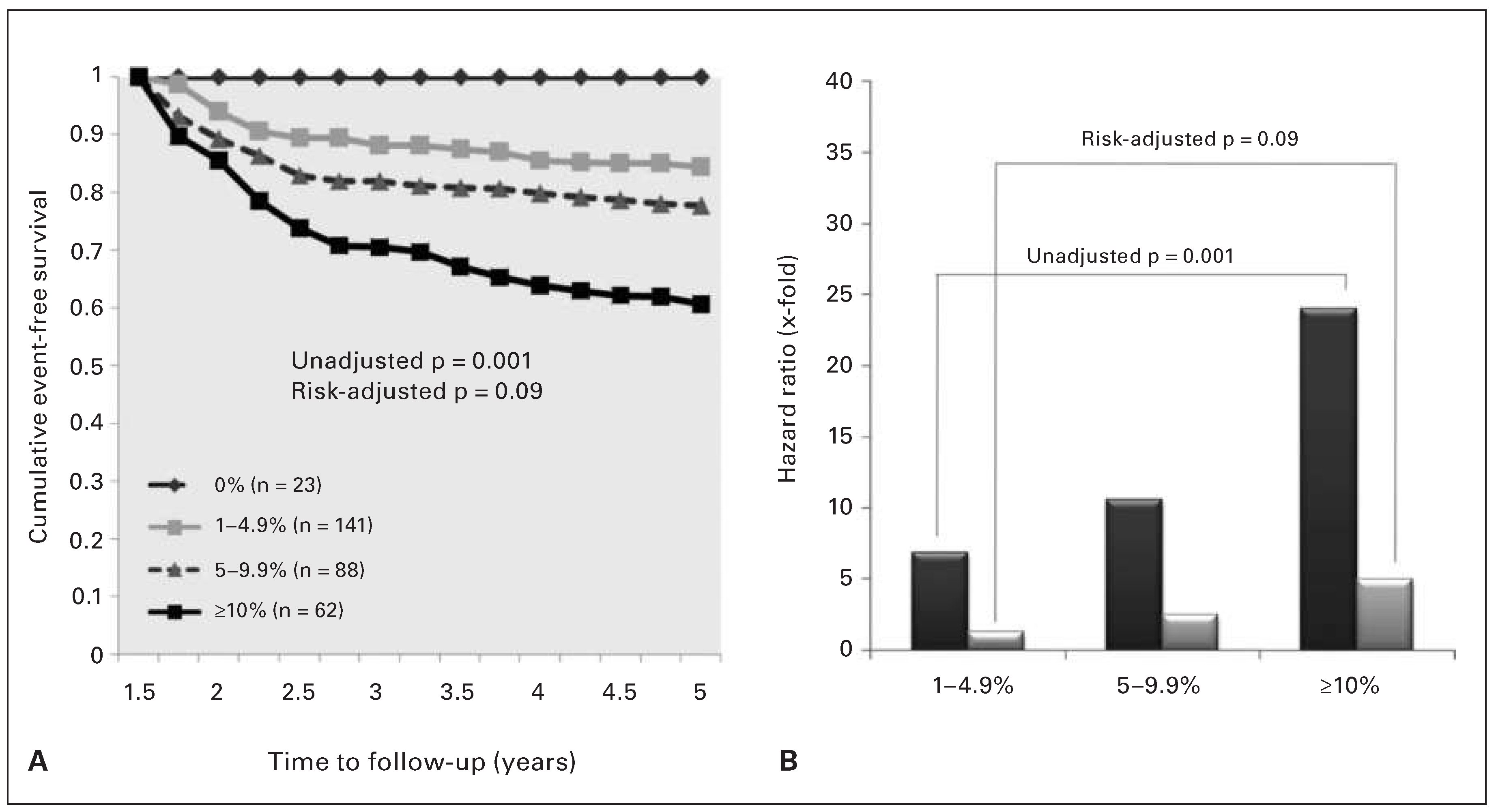
What is the Prognosis of Patients Revascularised on the Basis of FFR Measurement?
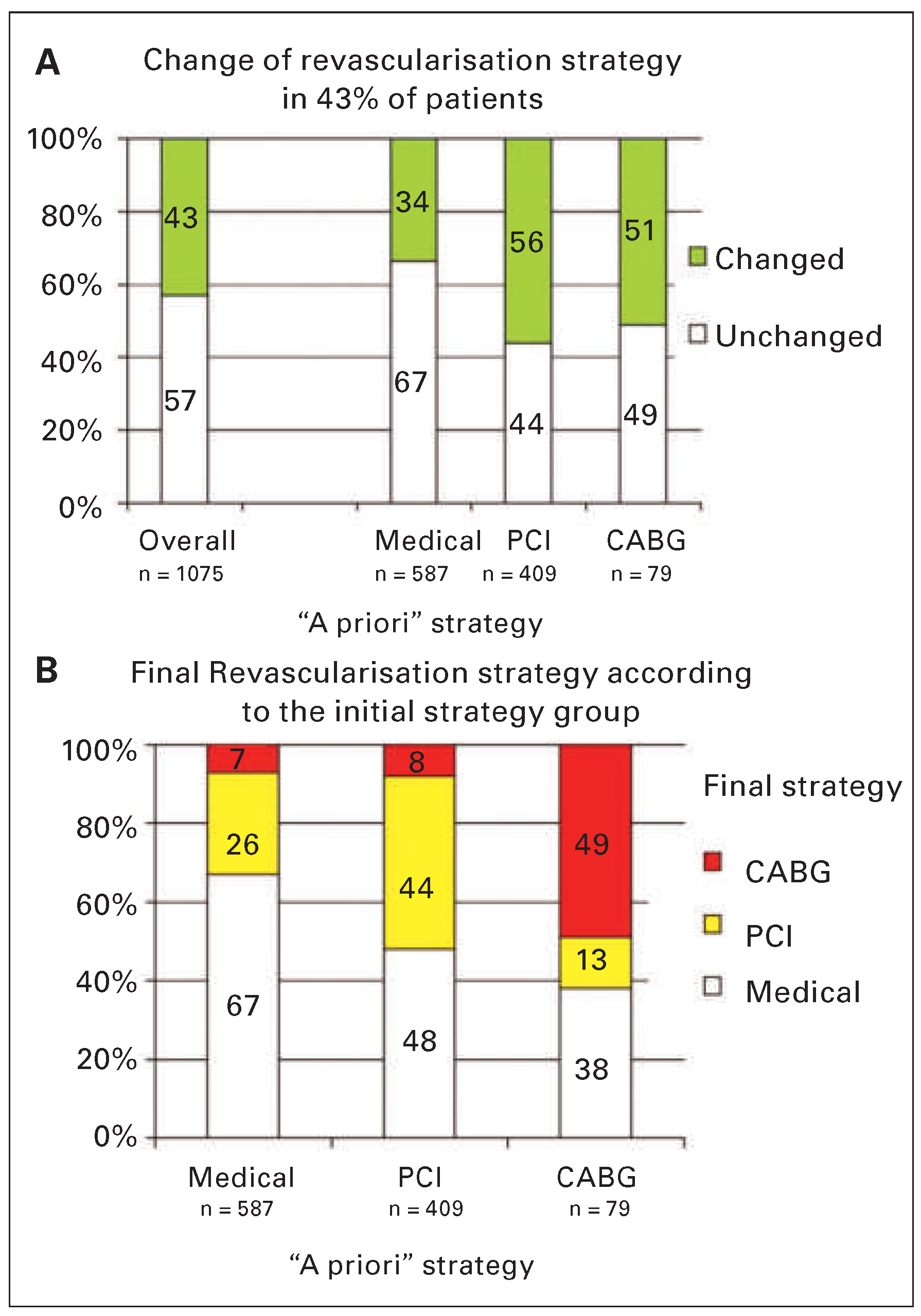
The Impact of FFR Measurement in Current PCI Strategies
iFR Versus FFR
Conclusion
Disclosure Statement
References
- Shaw, L.J.; Berman, D.S.; Maron, D.J.; Mancini, G.B.J.; Hayes, S.W.; Hartigan, P.M.; et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008, 117, 1283–91. [Google Scholar] [CrossRef] [PubMed]
- Hachamovitch, R.; Rozanski, A.; Shaw, L.J.; Stone, G.W.; Thomson, L.E.; Friedman, J.D.; et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress rest myocardial perfusion scintigraphy. Eur Heart J. 2011, 32, 1012–24. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011, 124, e574–651. [Google Scholar] [PubMed]
- Authors/Task Force m Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014, 35, 2541–2619. [Google Scholar]
- Pijls, N.H.; Van Gelder, B.; Van der Voort, P.; Peels, K.; Bracke, F.A.; Bonnier, H.J.; et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995, 92, 3183–93. [Google Scholar] [CrossRef] [PubMed]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007, 356, 1503–16. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.; Hamilos, M.; Pyxaras, S.; Mangiacapra, F.; Nelis, O.; De Vroey, F.; et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014, 35, 2831–8. [Google Scholar] [CrossRef] [PubMed]
- Pijls; et al. Fractional Flow Reserve Versus Angiography for Guiding Percutaneous Coronary Intervention in Patients With Multivessel Coronary Artery Disease: 2 Year Follow Up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) Study. J Am Coll Cardiol. 2010, 56, 177 84. [Google Scholar] [CrossRef]
- Van, Nunen; et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5 year follow up of a randomised controlled trial. Lancet. 2015. [Google Scholar]
- De Bruyne, B.; Fearon, W.F.; Pijls, N.H.; Barbato, E.; Tonino, P.; Piroth, Z.; et al. Fractional flow reserve guided PCI for stable coronary artery disease. N Engl J Med. 2014, 371, 1208–17. [Google Scholar] [CrossRef]
- Sen, S.; Escaned, J.; Malik, I.S.; Mikhail, G.W.; Foale, R.A.; Mila, R.; et al. Development and validation of a new adenosine independent index of stenosis severity from coronary wave intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012, 59, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, A.; Maehara, A.; Genereux, P.; Asrress, K.N.; Berry, C.; De Bruyne, B.; et al. Multicenter core laboratory comparison of the instantaneous wave free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. 2014, 63, 1253–61. [Google Scholar] [CrossRef] [PubMed]
| Study / participating Site | No. of lesions | Cut-off point | AUC from ROC | Overall accuracy in % |
|---|---|---|---|---|
| Total | 1523 | 0.9 | 0.81 | 80.4 |
| ADVISE | 432 | 0.91 | 0.82 | 81.9 |
| VERIFY | 654 | 0.89 | 0.8 | 79.4 |
| Seoul National University | 179 | 0.92 | 0.83 | 82.7 |
| Stony Brook University | 149 | 0.93 | 0.81 | 79.2 |
| Columbia University | 95 | 0.91 | 0.84 | 82.1 |
| AMC/VUMC/KCL | 84 | 0.9 | 0.78 | 78.6 |
© 2016 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Fournier, S.; Muller, O. The Impact of Fractional Flow Reserve and iFr on Current PCI Strategies. Cardiovasc. Med. 2016, 19, 67. https://doi.org/10.4414/cvm.2016.00390
Fournier S, Muller O. The Impact of Fractional Flow Reserve and iFr on Current PCI Strategies. Cardiovascular Medicine. 2016; 19(3):67. https://doi.org/10.4414/cvm.2016.00390
Chicago/Turabian StyleFournier, Stephane, and Olivier Muller. 2016. "The Impact of Fractional Flow Reserve and iFr on Current PCI Strategies" Cardiovascular Medicine 19, no. 3: 67. https://doi.org/10.4414/cvm.2016.00390
APA StyleFournier, S., & Muller, O. (2016). The Impact of Fractional Flow Reserve and iFr on Current PCI Strategies. Cardiovascular Medicine, 19(3), 67. https://doi.org/10.4414/cvm.2016.00390




