Presentation of the case
A 69-year-old male of Indian origin was referred for elective coronary angiography because of Canadian Cardiovascular Society class III angina pectoris and a recent acute coronary syndrome.
Our patient had a background of type 2 diabetes mellitus, hypertension and dyslipidaemia and was a chronic cigarette smoker. He first presented with angina in 2005, when severe disease in the mid left anterior descending coronary artery (LAD) was treated with a bare metal stent (BMS). Following further episodes of angina over the subsequent 12 months, he also received BMSs to both right coronary coronary artery (RCA) and circumflex coronary artery (Cx). Two years later, he again presented with worsening angina and, owing to the presence of diffuse severe three-vessel disease including significant in-stent restenosis (ISR) of the LAD, was referred for coronary artery bypass grafting. This was completed successfully using a pedicled left internal mammary artery (LIMA) anastamosed to the LAD and saphenous vein grafts (SVGs) anastomsed separately to both obtuse marignal and posterior descending arteries.
In 2011, he presented once again with angina. A computed tomography (CT) graft angiogram was undertaken, noting that both SVGs were occluded, with poor quality native vessels. However, the LIMA remained patent with good run-off. He underwent a viability magnetic resonance imaging (MRI) study, which demonstrated that the inferior wall was infarcted. At this point his left ventricular ejection fraction was 30%. He was therefore treated medically with escalating anti-anginal therapy.
He remained symptom-free until early 2014 when he presented with an acute coronary syndrome (troponin T 420 ng/l, reference range <30 ng/l) and ECG changes suggesting ischaemia in the anterolateral wall. At this time our patient was receiving maximal tolerated medical therapy: aspirin 100 mg o.d., clopidogrel 75 mg o.d., carvedilol 25 mg b.d., ivabradine 5 mg b.d., ISDN MR 60 mg o.d., simvastatin 40 mg o.d., furosemide 40 mg o.d., gliclazide MR 60 mg o.d., metformin 500 mg b.d. His creatinine was 103 μmol/l (estimated glomerular filtration rate 67 ml/min), haemoglobin 13.5 g/dl and weight 80 kg. His heart rate was 60 bpm and blood pressure 110/62 mm Hg.
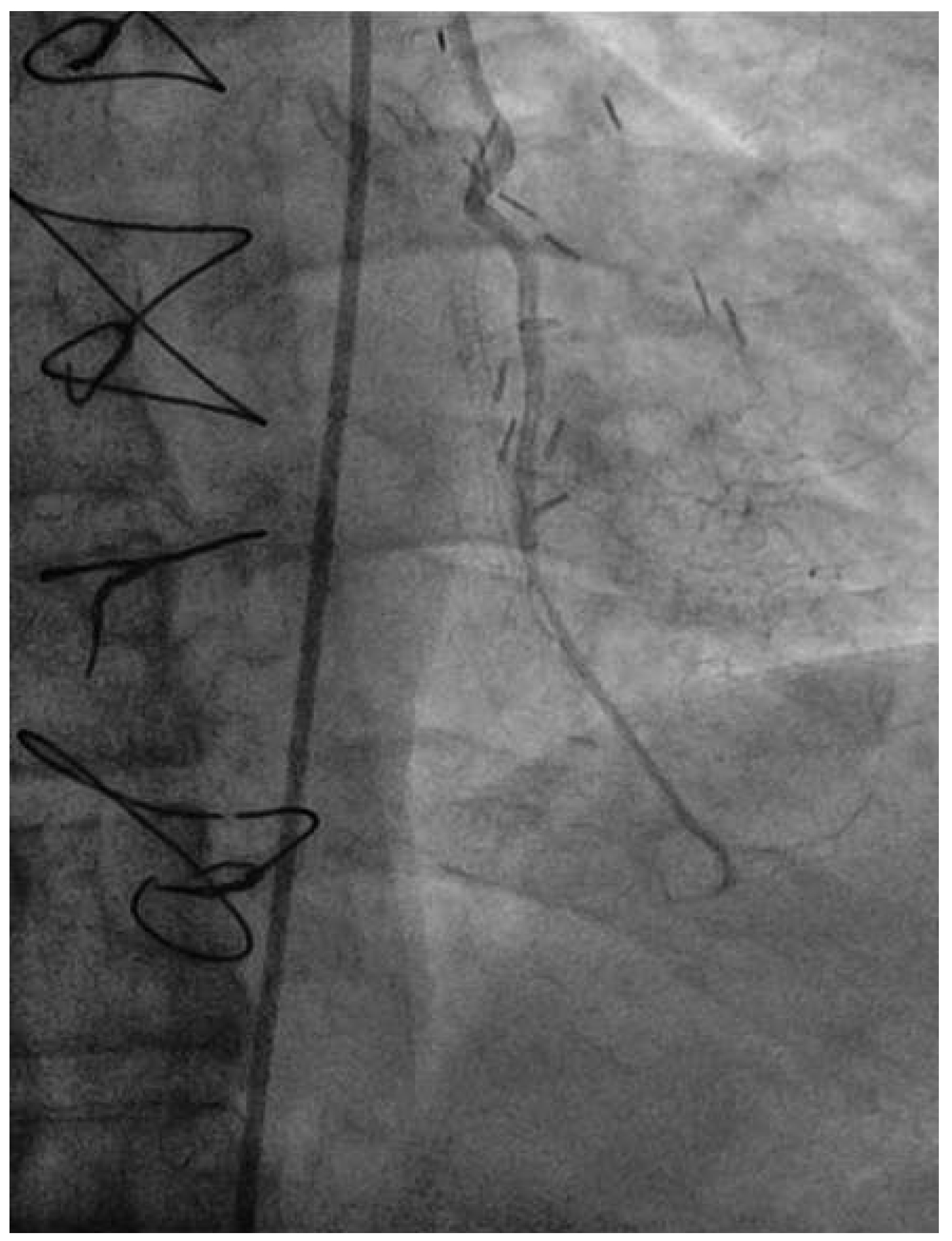
Coronary angiography of the left coronary system demonstrated severe left main stem (LMS) disease (
Figure 1). The severe LAD in-stent restenosis was very eccentric and appeared to be restricting the origin of a sizeable diagonal branch, which also had severe disease. Distally, the LAD was occluded at the point where another stent had been inserted previously and just beyond a large septal perforator. The Cx had severe ostial disease and gave rise to a heavily diseased first obtuse marginal branch. The remainder of Cx and right coronary artery (RCA) systems were occluded proximally, with absent collaterals. The only patent graft was the LIMA, anastomosed very distally to the LAD (
Figure 2). Following discussion, it was felt that neither Cx nor RCA were suitable targets for surgery and we therefore undertook intervention to the LMS and LAD / first diagonal bifurcation.
How did I treat this patient?
A 7-French XB3.5 guide catheter (Cordis, NJ, USA) was sited from the right femoral artery. The eccentricity of the in-stent restenosis within the LAD was considered likely to limit wire access to the diagonal branch, whilst the alternative strategy of direct balloon preparation of the LAD might lead to unfavourable plaque shift and further obscure the side-branch entry point. We therefore opted to undertake plaque modification using rotational atherectomy (RA) as our initial strategy, prior to wiring the diagonal and thereby also preserving carina position.
A 0.009″ floppy Rotawire (Boston Scientific, MA, USA) was advanced down the LAD into the second diagonal branch. Given that the bulk of the myocardium was dependent on the native LAD perfusion, a 5-F temporary pacing wire was sited prophylactically. Plaque modification within the LAD stent was performed using a 1.5 mm burr at 190 000 rpm using gentle constant pressure for a total of 10 s.
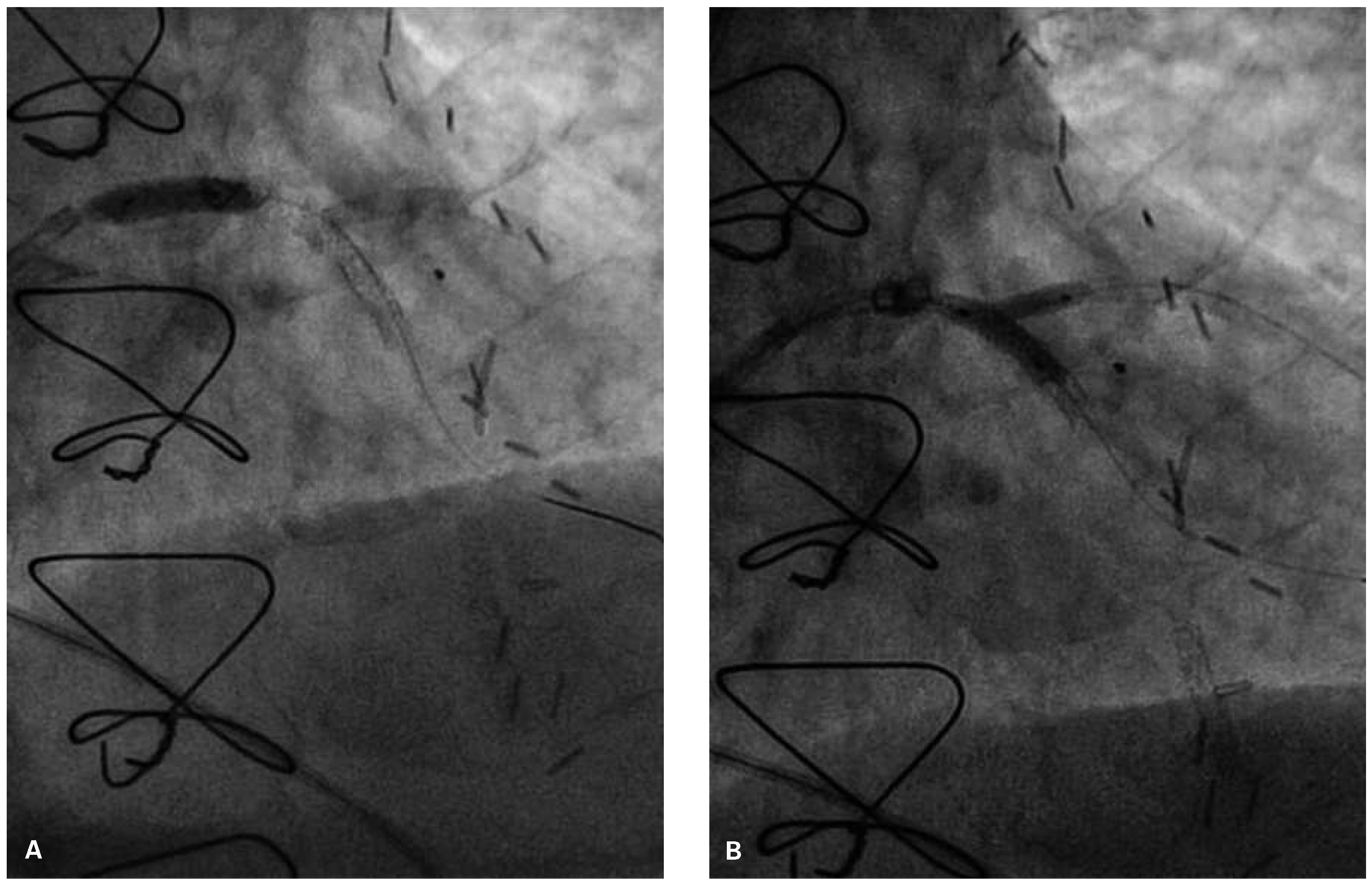
Following RA, both LAD and diagonal were wired with ease using separate Runthrough (Terumo, Japan) wires. Predilatation of both bifurcation limbs was undertaken using 1.5 × 15 mm Trek (Abbott) and 2.0 × 10 mm Sapphire (Orbus-Neich, Hong Kong, China) semicompliant balloons, which both expanded well at nominal pressure. Intending to perform an elective T-stent strategy, a 2.25 × 28 mm Promus Premier DES was deployed at 18 Atm in the diagonal branch, landing proximally at the ostium of this vessel (
Figure 3). After removal of the diagonal wire, a 3 × 38 Promus Premier stent (Boston Scientific) was sited from the origin of the LMS across the first diagonal and deployed at 18 Atm (
Figure 4). The diagonal branch was then rewired with the runthrough wire. A kissing inflation was performed at 12 Atm using a 3.5 × 12 Quantum (Boston Scientific) and 2.5 × 12 Sapphire noncompliant (NC) balloons in LAD and diagonal, respectively (
Figure 5A). The proximal LAD and LMS were then optimised using the 3.5 Quantum NC (LAD) and a Hiryu (Terumo) 4 × 10 NC (LMS) balloons at 22 and 18 Atm, respectively (
Figure 5B). The final angiographic result was excellent (
Figure 6). Intravascular ultrasound of the LMS was not undertaken as angiographically the LMS stent already appeared completely expanded and oversized. We opted not to intervene in the ostial left Cx in order to best preserve the geometry of the LMS stent.
Haemostasis was achieved using manual digital compression 6 hours after heparin. The total fluoroscopy time was 16.4 min, total contrast 110 ml Iopamiro 370 (Bracco S.p.A., Feretinoa, Italy). Our patient was returned to the ward for overnight observation and discharged home the following day. He remains symptom-free at 3-month follow-up.
Discussion
This was a complex case, undertaken in a patient with severe native coronary artery disease who had previous unsatisfactory surgical and percutaneous attempts at revascularisation. RA was used electively in this case to modify eccentric severe in-stent restenosis preventing wire entry into an important diagonal branch. This strategy allowed the remainder of the PCI procedure to be completed successfully and without immediate complication.
Although contemporary guidewires have excellent performance, side-branch access in situations such as the present case can prove troublesome [
1]. The use of a floppy hydrophilic wire, such as the Whisper LS (Abbott, IL, USA) with a double curved tip, can with persistence and careful manipulation prove successful. Unfortunately, due to the eccentricity of ISR, the angle of entry to the diagonal in this case was around 270° and therefore alternative strategies need to be considered. In this situation, the reverse wiring technique can be used successfully [
2]. Here a hairpin bend is formed on the coronary guidewire about 4–5 cm from its distal tip. The main vessel is then wired beyond the side-branch and on wire pullback, the floppy tip is used to engage the side-branch. Alternatively, specific sidebranch access tools have been developed. These include the Crusade (Kaneka, Japan) double lumen microcatheter and the Venture (St Jude, MN, USA) deflectable tip catheter, which may prove useful in appropriate situations, although neither is stocked routinely in our catheter laboratory [
3]. Interestingly, the Crusade (and other) side-branch access catheters have also been successfully used in conjunction with the reverse wire technique to deliver the hairpin of the wire beyond a tight main vessel stenosis. This is important to note as the severe LAD stenosis may have precluded conventional use of the reverse wire technique [
4].
Where direct wiring techniques fail, the usual alternative is balloon dilatation of the main vessel to achieve plaque modification and facilitate easier side-branch entry. However, plaque shift can be unpredicatable and, if unfavourable, this may result in abrupt closure of the side branch, hampering further wiring efforts. An alternative strategy to be considered is elective RA, which may debulk the plaque in a more controlled manner and can help preserve side-branch entry [
5].
RA was developed in the early 1980s as a strategy to debulk and modify coronary plaque disease, particularly in the presence of extensive calcification. RA relies upon the differential properties of the various components of coronary plaque disease, resulting in “cutting” of the harder plaque components (e.g., calcium), whilst leaving the elastic vessel wall largely unharmed. Although useful for this purpose, RA fell out of favour as the “over-the-wire” technique requires two experienced operators, and clinical trials (largely in the bare metal stenting era) failed to demonstrate improvements in either mortality or morbidity [
6]. However, recently RA has again emerged as an important ancillary tool to aid stent delivery into complex anatomy as marked improvements in stent technology have allowed complex PCI such as in the present case to be undertaken with good outcomes.
Regarding in-stent restenosis, usual practice is to undertake predilatation with noncompliant and/or cutting balloons and to ensure adequate expansion prior to further stent (or drug-eluting balloon) deployment. This is important as failure to adequately expand during the predilatation stage is strongly predictive of recurrent in-stent restenosis [
7]. RA has previously been undertaken through previously deployed stents without complication and may be useful in cases where aggressive predilation has failed to achieve adequate expansion. However, as yet RA has not been demonstrated to reduce likelihood of recurrent in-stent restenosis [
8]. Therefore the utility of routine RA in the context of instent restenosis is uncertain, particularly as such disease is relatively elastic and may not always respond to RA in the same way as calcific atheroma.
Conclusion
RA can be used effectively to modify in-stent restenosis and safely permit wire access in a difficult-to-wire side-branch.