Introduction
Mitral regurgitation (MR) is the most frequent valvular heart disease in developed countries [1]. MR can be organic (resulting from primary anatomical alterations affecting the valve leaflets or subvalvular apparatus: primary MR) or functional (resulting from left ventricular [LV] remodelling processes causing papillary muscle dislocation and leaflet tethering, in the absence of structural abnormalities of the valve: secondary MR).
The natural history of severe MR is unfavourable, leading to worsening LV failure, pulmonary hypertension, atrial fibrillation and death [2].
The most common aetiology of organic MR in Western countries is degenerative MR (DMR). Among organic diseases, MitraClip can be used to treat only those with a degenerative aetiology that is due to a leaflet tissue alteration known as myxomatous degeneration or fibroelastic deficiency, leading to mitral valve (MV) prolapse or flail [3].
Secondary MR is the consequence of LV dysfunction and dilation due to a maladaptive process in the context of postischaemic or idiopathic dilated cardiomyopathy.
Surgical repair represents the optimal treatment for severe DMR because of its well-documented advantages over valve replacement in terms of perioperative mortality, preservation of postoperative LV function and long-term survival [4,5]. Indeed, if performed before the onset of limiting symptoms or the development of LV dysfunction, MV repair is able to restore normal life expectancy and quality of life [6]. By contrast, surgical correction of functional MR (FMR) is controversial, because the prognosis of the patient is related more to the cardiomyopathic process than to MR. Outcomes after surgical correction of FMR remain suboptimal in many cases, and perioperative mortality is not negligible [7–10].
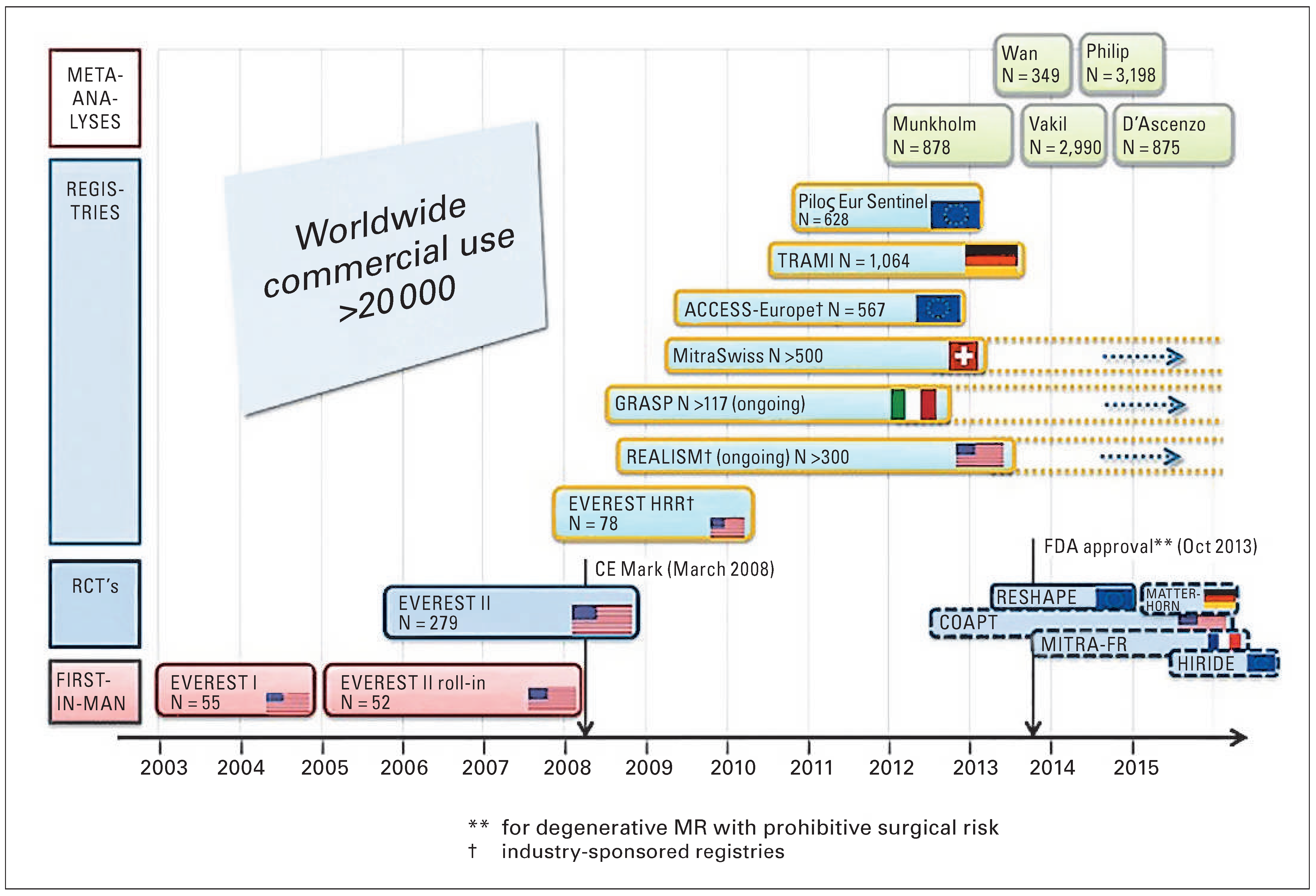
The Euro Heart Survey conducted by the European Society of Cardiology (ESC) showed that up to 50% of patients with severe MR are today denied surgical treatment because they are felt to be at too high risk for surgery owing to advanced age or comorbidities [11]. Therefore, over the past few years, new transcatheter techniques have been developed to treat MR with less invasive approaches. Different types of transcatheter procedures are becoming available. Currently, the procedure with the widest clinical experience is the percutaneous edge-to-edge technique performed with the MitraClip System (Abbott Park, IL, USA). Up to now, over 20 000 patients have been treated worldwide, with an increasing number of procedures in Europe and in Switzerland (
Figure 1 and
Figure 2).
The EVEREST study (Endovascular Valve Edge-to-Edge Repair of Mitral Regurgitation Study) comprised a series of trials, including the first randomised controlled trial in which percutaneous treatment was compared with surgical treatment in selected patients with MR (mainly of degenerative aetiology). The study results indicated that, 1 year after the procedure, surgery was superior to percutaneous treatment in terms of efficacy (measured as freedom from recurrence of MR and survival), whereas the percutaneous treatment was associated with higher safety. In a post-hoc analysis, MitraClip therapy appeared to be noninferior to surgery in terms of effectiveness in three subgroups of patients: patients older than 70 years, those with LV dysfunction and those with FMR [12–26]. Final results of the EVEREST trial showed that both MitraClip and mitral valve surgery are durable through 5 years of follow-up. MitraClip was associated with sustained reduction in MR, improvement in LV volumes and dimensions and improvement in New york Heart Association (NYHA) functional class. Regardless of MR aetiology, few MitraClip patients were converted to MV surgery beyond the 1-year primary endpoint. Interestingly, mitral annular dimension did not increase after Mitra- Clip after 5 years in both DMR and FMR (T. Feldman. Final Results of the EVEREST II Randomized Controlled Trial of Percutaneous and Surgical Reduction of Mitral Regurgitation. Washington, ACC 2014).
Primary and secondary MR are very different entities in terms of aetiology, prognosis,and management and they will therefore be discussed separately in this review.
The bibliographic research was conducted using the MEDLINE (PubMed) database. Key words used for the research included: “mitraclip”, “percutaneous edge to edge”, “mitraclip registries”, “mitraclip degenerative”, “mitraclip functional”, “complications following mitraclip”, alon and in combination. Only information from full-text articles in English from international medical journals was considered. To ensure that the clinical information was as current as possible, abstracts from the most recent and important international interventional cardiology meetings were considered.
Degenerative mitral regurgitation
The transcatheter device with the widest experience in organic MR is the MitraClip therapy. Currently, most patients undergoing the MitraClip procedure are non-surgical high-risk candidates. Data from the EVEREST trials and registries in Europe and the USA suggest that the MitraClip technique has a procedural success rate (postprocedural MR ≤2+) of around 75%, is relatively safe and generally well-tolerated, even by patients in poor clinical condition [12–26]. According to the ESC 2012 Guidelines [27], the percutaneous edge-to-edge procedure may be considered in patients with symptomatic severe DMR who fulfil the echocardiographic criteria of eligibility, are judged inoperable or at high surgical risk by a “heart team”, and have a life expectancy greater than 1 year (class IIb, level of evidence C). High surgical risk patients rejected from the EVEREST II trial were included into two prospective registries, the EVEREST II High-risk Registry (EVEREST HRR) and the ongoing REALISM High-Risk Registry. Subjects enrolled in these two registries had a Society of Thoracic Surgeons (STS) risk >12% or additional risk factors qualifying the patient as high risk. A degenerative aetiology was present in 105 patients. Thirty-day mortality was 6.7%. At 1 year, 85% of the DMR patients had sustained a reduction in MR; 87% of the patients improved to an NYHA functional class I/II at 1 year. The annual rate of hospitalisation for heart failure was significantly reduced compared with baseline after MitraClip implantation. Sustained clinical benefit is present over 5 years’ follow-up [28].
The ACCESS-EU study is the first large database reporting outcomes of the MitraClip in a high-risk population of patients. The study had no prespecified criteria for treatment and better reflects real- world practice: whereas most of the patients enrolled in the EVEREST trials were surgical candidates, the majority of the patients treated in the ACCESS-EU are high surgical risk patients [29].
In the ACCESS-EU study, out of 567 patients undergoing the MitraClip procedure, 117 patients (20%) had a DMR aetiology [30]. The overall DMR cohort was elderly (75.6 ± 12.1 years) with 61.5% of patients over 75 years of age and 49.6% male. The majority of ACCESS-EU Phase I DMR patients presented multiple comorbidities at baseline. Approximately one quarter (23.9%) of the patients had had previous cardiovascular surgery including coronary artery bypass grafting (17.1%) and 27.6% of patients had undergone percutaneous coronary intervention prior to enrolment in the ACCESS-EU study. The vast majority of patients (96.6%) in the DMR cohort had a mitral regurgitation grade 3+ or 4+ at baseline and most (73.1%) were symptomatic with NYHA functional class III or IV. Mean logEuroSCORE I for the entire DMR cohort was 15.5 ± 13.3%. Stratification into high- and low-risk patients revealed important demographic differences culminating in mean log-EuroSCORE I of 33.1 ± 11.5% and 8.6 ± 5.1%, respectively for the two cohorts.
Procedural success was 94.9%. Overall, mean length of stay in the intensive care unit, cardiac care unit or postanesthesia care unit following the MitraClip procedure was 2.3 ± 2.6 days with a median of 1 day, and no differences between high-risk and low-risk DMR patients. However, the median postprocedural hospital stay was slightly longer for high surgical risk patients when compared with low surgical risk patients (7.2 ± 4.3 days for high risk vs 6.5 ± 5.5 days for low risk). Also, a significantly larger proportion of low-risk patients were discharged home with or without home health care than of high-risk patients (82.9% and 74.2% respectively, p = 0.003) [30]. About 90% of patients achieved an MR reduction to grade ≤2+ at discharge, and 60% achieved an MR reduction to grade ≤1+ at discharge.
The incidence of perioperative adverse events was low (stroke 0.9%, myocardial infarction 0.9%, acute renal failure 2.6%, bleeding complication 3.4%, mitral valve surgery 1.7%). Thirty-day mortality was 6.0% (9.1% and 4.8% for high- and low-risk subgroups, respectively). Causes of death were classified as cardiac in 42.9%.
Overall rate of freedom from MR of grade >2+ was 75% at 12 months. Meaningful clinical improvements were observed: 80.8% of the patients were in NYHA functional class I–II at 1-year follow-up. Both Minnesota Living with Heart Failure questionnaire (MLHFQ) scores and 6-minute walking test distance improved significantly at 12 months compared with baseline. Overall mortality at 1 year was 17% [30].
The results of the ACCESS-EU DMR study showed that MitraClip therapy might serve as a complementary nonsurgical therapeutic option for DMR patients who are considered at high risk or ineligible for surgery, providing significant reductions in MR and improvements in clinical outcomes at 12 months in selected high-risk patients with DMR.
Lim et al. recently reported the mid-term results of 127 DMR patients with prohibitive surgical risk who underwent MitraClip treatment. “Prohibitive risk” includes patients with a STS risk calculator predicted risk of mortality for mitral valve replacement of ≥8%, or if the patient has factors for prohibitive surgical risk not included in the STS risk calculator. Patients were elderly (mean age 82 years), severely symptomatic (87% NYHA Class III–IV), with an STS mortality score of 13.2 ± 7.3%. MitraClip was successfully implanted in 95%. Hospital stay was about 3 days. Thirty-day mortality was 6.3%. Periprocedural adverse event rate was low, including myocardial infarction in 0.8%, stroke in 2.4%, acute renal failure 1.6% and major vascular complications in 5.5% [31].
One-year mortality was 23.6%. The majority of surviving patients (82.9%) remained MR ≤2+ at 1 year and 86.9% were in NYHA functional class I–II. left ventricular end-diastolic volume decreased (125.1 ± 40.1 ml to 108.5 ± 37.9 ml, p <0.0001). SF-36 quality-of-life scores improved, and hospitalisations for heart failure were reduced in patients whose MR was reduced. The conclusions from the study are that MitraClip treatment in prohibitive surgical risk patients is associated with safety and good clinical outcomes, including rehospitalisation decrease, functional improvements and favourable ventricular remodelling at 1 year.
Good clinical outcomes in the setting of DMR elderly patients were reported by Taramasso et al. in a single centre experience [32]. Forty-eight high-risk consecutive patients with severe DMR underwent MitraClip implantation (mean age 78.5 years; 57% of the patients were older than 80 years). Mean STS score was 12% and 71% were in NYHA class III or IV. The device was successfully implanted in 47 of 48 patients (98%). In-hospital mortality was very low (1/48 patients, 2%). Perioperative major events included 2% acute renal failure and 4% bleeding complication, while no cerebrovascular accident or acute myocardial infarction was reported. The median intensive care unit stay was 22 hours. Predischarge echocardiography showed a mitral regurgitation reduction to grade 2+ or less in 43 of 47 patients (91.5%). Actuarial survival was 89% and 70.2% at 1 and 2 years, respectively (82% in patients aged <80 years and 95% in patients older than 80 years at 1 year). Freedom from mitral regurgitation 3+ or greater was 80% at 1 year and 76.6% at 2 years. At 1 year, 93% of survivors were in NYHA class I–II (100% of patients aged <80 years and 88% of patients aged ≥80 years). Significant quality-of-life and functional status improvements were documented at follow-up.
A significant improvement in perceived quality of life after MitraClip therapy for DMR was also documented by Ussia et al. [33] in a small series of 14 consecutive high-risk patients with DMR who reported both physical and mental status improvement 6 months after the procedure. The low rate of postprocedural complications with MitraClip, owing to the absence of the surgical trauma, did not prolong the recovery time and did not have a significant impact on the quality of life in the elderly and high-risk population. Patients who have received the MitraClip demonstrate improved physical and mental quality-of-life scores.
In this scenario, transcatheter edge-to-edge treatment of DMR could play a relevant clinical role in the near future. Several reports suggest that the reduction in invasiveness of the transcatheter edge-to-edge repair is associated with low procedural risk and significant clinical benefit, and this can be beneficial in the subset of the very old and high-risk populations. This is particularly true in patients with associated cardiac conditions (i.e., associated coronary artery disease or atrial fibrillation), because transcatheter interventions offer the unique opportunity of staging interventions to mitigate risk. In October 2013, the MitraClip received official approval by the USA Food and Drug Administration (FDA) for use in symptomatic patients with degenerative mitral regurgitation and with prohibitive surgical risk. Although we are nowadays already treating many older patients with comorbidities with MitraClip instead of conventional surgery, there is a lack of evidence in this particular patient population and it is debatable which is the optimal treatment option. The upcoming HiRiDe Trial, which is going to randomise intermediate to high-risk patients older than 75 years with severe DMR to MitraClip or conventional mitral surgery, has been designed to provide solid evidence in this context.
Functional mitral regurgitation
The use of transcatheter edge-to-edge repair to treat isolated FMR has exactly the same level of evidence and class of indication as surgical treatment, reflecting a similar lack of evidence to support a more aggressive treatment strategy. In 2012, MitraClip was recommended by both the ESC Heart Failure and ESC/EACTS guidelines on valvular heart disease [27] for patients with symptomatic severe secondary MR despite optimal medical therapy (including cardiac resynchronisation therapy if indicated), with anatomical suitability, who are judged inoperable or at high surgical risk by a team of cardiologists and cardiac surgeons, and with a life expectancy greater than 1 year (recommendation class IIb, level of evidence C).
At the moment, there are three ongoing randomised trials comparing MitraClip and optimal medical therapy in patients with severe FMR (the COAPT trial in the USA and the RESHAPE and the MITRA-FR trials in Europe), while one randomised trial, the MATTERHORN, is comparing MitraClip to surgical treatment (
Figure 3).
MitraClip therapy has been performed to treat FMR in high-risk and end-stage patients with favourable safety and efficacy results. Several clinical experiences with satisfactory acute and mid-term results in FMR are now reported in the literature.
The High-Risk Registry of the EVEREST II study is the first to suggest a potential prognostic benefit in highrisk patients treated with the MitraClip in both the degenerative and functional MR groups. Patients treated with the MitraClip had a better survival rate at 1 year than the survival observed in a comparator-matched group. In addition, the registry has demonstrated a significant reduction in heart failure hospitalisation by a factor of approximately 50% as compared with the year before implantation, improvement in clinical symptoms, and significant LV reverse remodelling over 12 months in patients submitted to MitraClip therapy [28].
The ACCESS-EU registry [29] offers a snapshot of the characteristics of patients who currently undergo the procedure in the European postmarketing real world: they are mainly elderly patients with comorbidities, at high surgical risk or who are inoperable, and with a high prevalence of FMR (more than 70% of the total). The reported mortality at 30 days was 3.4%, which is notably low, especially if we consider that the majority of patients were at high surgical risk (Logistic Euro- SCORE 23 ± 18%) and affected by FMR secondary to chronic heart failure.
At 12 months, the survival rate was 82%, and 79% of patients showed a residual MR less than or equal to 2+. Although this degree of reduction is lower than that observed after surgical repair, the persistence of an MR grade less than or equal to mild-to-moderate could be a reasonable therapeutic target in patients at high surgical risk. Moreover, the ACCESS registry demonstrated a remarkable clinical effectiveness: at 1 year after the procedure, 71% of patients were in NYHA functional class I or II and most patients have an improvement in quality of life and a gain in functional capacity.
Satisfactory clinical results have also been reported by the German transcatheter mitral valve interventions (TRAMI) registry [34] that enrolled 1 064 patients (525 patients ≥76 years and 539 patients <76 years; more than 70% with FMR). Age was the most frequent reason for nonsurgical treatment in the elderly. The rate of in-hospital major cardiac and cerebrovascular events (MACCE: death, myocardial infarction, stroke) was low in both groups (3.5% vs 3.4%) and the proportion of nonsevere mitral regurgitation at discharge was similar (95.8% vs 96.4%, p = 0.73). A logistic regression model did not reveal any significant impact of age on acute efficacy and safety of MitraClip therapy, showing that elderly and younger patients have similar clinical benefits.
The Transcatheter Valve Treatment Sentinel Pilot Registry [35], a prospective, independent, consecutive collection of individual patient data, enrolled a total of 628 patients (mean age 74.2 ± 9.7 years) who underwent a transcatheter edge-to-edge procedure between January 2011 and December 2012 in 25 centres in 8 European countries. The prevalent pathogenesis was FMR (72.0%). The majority of patients (85.5%) were highly symptomatic (NYHA functional class III or higher), with a high logistic EuroSCORE (20.4 ± 16.7%). Acute procedural success was high (95.4%) and similar in FMR and DMR. In-hospital mortality was low (2.9%) and the estimated 1-year mortality was 15.3%, which was similar for FMR and DMR. The estimated 1-year rate of rehospitalisation because of heart failure was 22.8%. Paired echocardiographic data from the 1-year follow-up, available for 368 consecutive patients, showed a persistent reduction in the degree of mitral regurgitation at 1 year, with 6.0% of patients with severe mitral regurgitation.
The prospective MitraSwiss registry enrolled the first 100 consecutive high-risk patients treated with Mitra- Clip in Switzerland between March 2009 and April 2011 (62 patients had FMR). Acute procedural success was achieved in 85% of patients, with an overall 12 months’ survival of 84.6% [36]. The 2-year results were recently reported for 74 patients: a baseline mean transmitral gradient <3 mm Hg, a left atrium size <50 ml/m, the absence of obstructive pulmonary disease and of chronic renal failure, and reduction of MR to less than moderate were associated with favourable outcome [37]. Additionally, haemodynamic measurements documented a significant reduction in pulmonary pressure with a concomitant increase in cardiac index after MitraClip therapy [38].
MitraClip therapy has been demonstrated to be feasible and safe even in critically ill patients (NYHA IV), leading to symptomatic improvement in over twothirds of these patients; however, it is associated with an elevated 30-day mortality, compared with stable clinical conditions [39].
Table 1,
Table 2 and
Table 3 summarise the baseline profiles and outcomes of the most important “real-world” registries.
Other applications
It should be briefly mentioned that the transcatheter edge-to-edge has been recently used to successfully address LVOT obstruction due to mitral systolic anterior motion (SAM) in the context of HOCM, showing that SAM-induced obstruction might be a valuable target for the MitraClip [40].
Conclusions
Although surgical repair remains the gold standard therapy, transcatheter edge-to-edge treatment of DMR could play a relevant clinical role in the near future, especially in the subset of the very old and high-risk population. Randomised trials comparing MitraClip and surgery in specific high-risk groups of patients would help to clarify the future role of the MitraClip in DMR. Regarding FMR, now that the safety of the percutaneous edge-to-edge procedure has been proved, Mitra- Clip is considered as an alternative option for patients at high surgical risk. Some issues regarding efficacy and long-term durability have to be addressed in order to reduce the threshold of risk and expand Mitraclip therapy to lower-risk population. Final results of the EVEREST II trial showed that when acute optimal results are achieved with MitraClip, durability up to five years is usually guaranteed. MitraClip therapy is not so far a palliative therapy, when properly performed. This implies correct patient selection and timing, advanced procedural imaging and optimal procedural performance [41].
In future years, MitraClip has probably the potential to become a first-line option in patients with isolated FMR. Surgery will still be considered in intermediate-risk patients, particularly when associate conditions (such as atrial fibrillation and coronary artery disease) are present. However, we need to treat patients at an earlier stage if we look for a significant prognostic benefit and in this context a heart team approach will be mandatory.
The definition of a specific and shared educational training programme of an interventional mitral valve operator (including both surgical and interventional skills) will represent a major challenge for the cardiovascular community in the next years.