Catheter Ablation for Atrial Fibrillation in a Real-World Setting
Abstract
Introduction
Methods
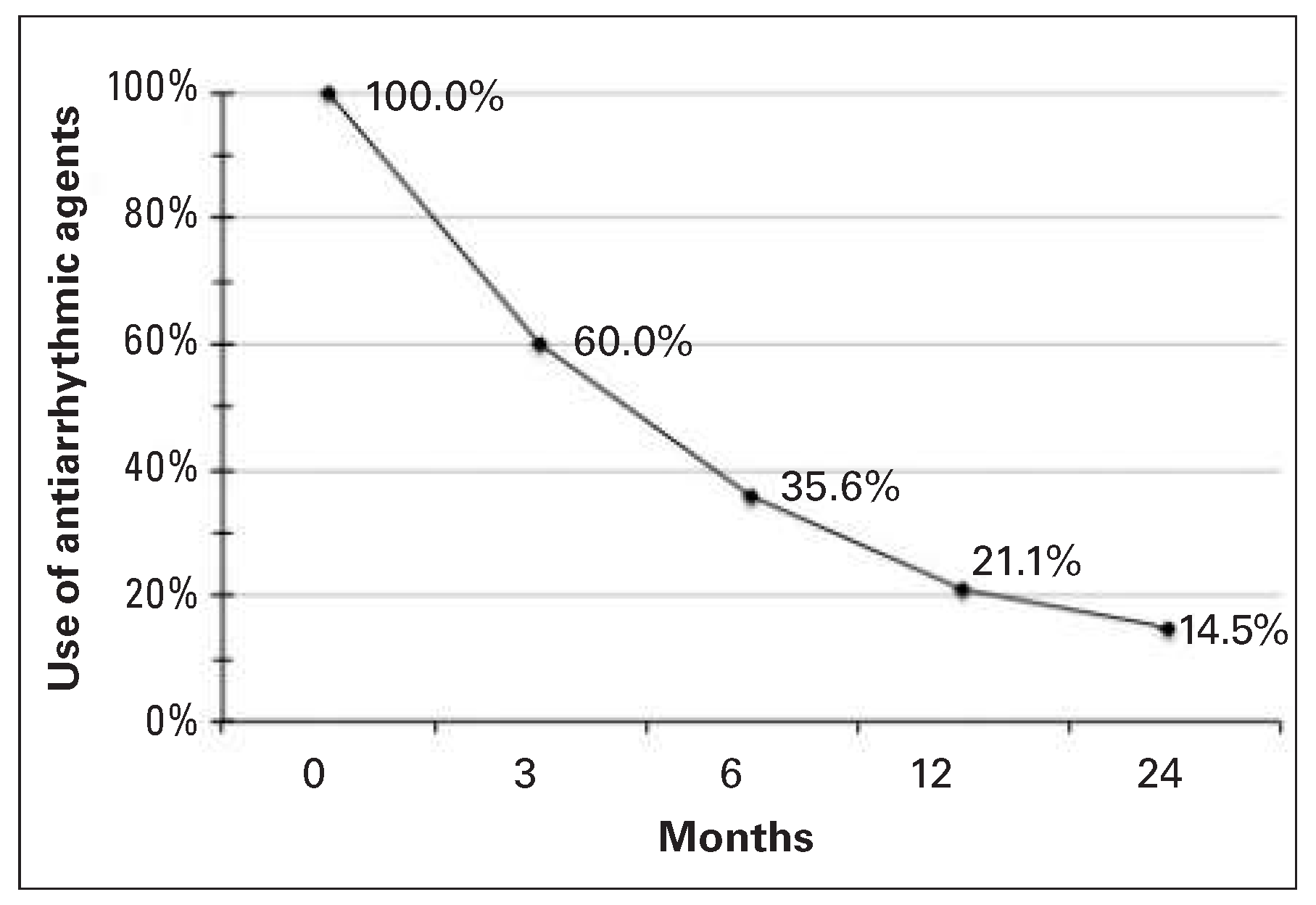
Results
Discussion
Limitations
Conclusions
Conflicts of Interest
References
- Feinberg, W.M.; Blackshear, J.L.; Laupacis, A.; Kronmal, R.; Hart, R.G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995, 155, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; Chen, S.A.; et al. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: A report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the european heart rhythm association (ehra), a registered branch of the european society of cardiology (esc) and the european cardiac arrhythmia society (ecas); and in collaboration with the american college of cardiology (acc), american heart association (aha), the asia pacific heart rhythm society (aphrs), and the society of thoracic surgeons (sts). Endorsed by the governing bodies of the american college of cardiology foundation, the american heart association, the european cardiac arrhythmia society, the european heart rhythm association, the society of thoracic surgeons, the asia pacific heart rhythm society, and the heart rhythm society. Heart Rhythm 2012, 9, 632–696.e21. [Google Scholar]
- Camm, A.J.; Lip, G.Y.H.; De Caterina, R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; et al. 2012 focused update of the esc guidelines for the management of atrial fibrillation: An update of the 2010 esc guidelines for the management of atrial fibrillation * developed with the special contribution of the european heart rhythm association. Eur. Heart J. 2012, 33, 2719–2747. [Google Scholar]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cleveland, J.C., Jr.; Cigarroa, J.E.; et al. 2014 aha/acc/hrs guideline for the management of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. Circulation 2014, 130, e199–e267. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Jais, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Arentz, T.; Haegeli, L.; Sanders, P.; Weber, R.; Neumann, F.J.; Kalusche, D.; et al. High-density mapping of spontaneous pulmonary vein activity initiating atrial fibrillation in humans. J Cardiovasc Electrophysiol. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Haegeli, L.M.; Calkins, H. Catheter ablation of atrial fibrillation: An update. Eur Heart J. 2014, 35, 2454–2459. [Google Scholar] [CrossRef]
- Haegeli, L.M. Cardiopulse. Percutaneous radiofrequency catheter ablation of atrial fibrillation. Eur Heart J. 2012, 33, 2625–2627. [Google Scholar]
- Haegeli, L.M.; Kotschet, E.; Byrne, J.; Adam, D.C.; Lockwood, E.E.; Leather, R.A.; et al. Cardiac injury after percutaneous catheter ablation for atrial fibrillation. Europace 2008, 10, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Haegeli, L.M.; Wolber, T.; Ercin, E.; Altwegg, L.; Krasniqi, N.; Novak, P.G.; et al. Double transseptal puncture for catheter ablation of atrial fibrillation: Safety of the technique and its use in the outpatient setting. Cardiol Res Pract. 2010, 2010, 295297. [Google Scholar] [CrossRef] [PubMed]
- Wutzler, A.; Wolber, T.; Parwani, A.S.; Huemer, M.; Attanasio, P.; Blaschke, F.; et al. Robotic ablation of atrial fibrillation with a new remote catheter system. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2014, 40, 215–219. [Google Scholar] [CrossRef]
- Haegeli, L.M.; Duru, F.; Lockwood, E.E.; Luscher, T.F.; Sterns, L.D.; Novak, P.G.; et al. Feasibility and safety of outpatient radiofrequency catheter ablation procedures for atrial fibrillation. Postgrad Med J. 2010, 86, 395–398. [Google Scholar] [CrossRef]
- Calkins, H.; Reynolds, M.R.; Spector, P.; Sondhi, M.; Xu, Y.; Martin, A.; et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: Two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009, 2, 349–361. [Google Scholar] [CrossRef]
- Wazni, O.M.; Marrouche, N.F.; Martin, D.O.; Verma, A.; Bhargava, M.; Saliba, W.; et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: A randomized trial. JAMA 2005, 293, 2634–2640. [Google Scholar] [CrossRef]
- Pappone, C.; Augello, G.; Sala, S.; Gugliotta, F.; Vicedomini, G.; Gulletta, S.; et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: The apaf study. J Am Coll Cardiol. 2006, 48, 2340–2347. [Google Scholar] [CrossRef]
- Jais, P.; Cauchemez, B.; Macle, L.; Daoud, E.; Khairy, P.; Subbiah, R.; et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: The a4 study. Circulation 2008, 118, 2498–2505. [Google Scholar] [CrossRef]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; De Paola, A.; Marchlinski, F.; Natale, A.; et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA 2010, 303, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Oral, H.; Pappone, C.; Chugh, A.; Good, E.; Bogun, F.; Pelosi, F., Jr.; et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006, 354, 934–941. [Google Scholar] [CrossRef]
- Cosedis Nielsen, J.; Johannessen, A.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Kongstad, O.; et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012, 367, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Verma, A.; Connolly, S.J.; Kuck, K.H.; Nair, G.M.; Champagne, J.; et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (raaft-2): A randomized trial. JAMA 2014, 311, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Noheria, A.; Kumar, A.; Wylie, J.V., Jr.; Josephson, M.E. Catheter ablation vs antiarrhythmic drug therapy for atrial fibrillation: A systematic review. Arch Intern Med. 2008, 168, 581–586. [Google Scholar] [CrossRef]
- Brooks, A.G.; Stiles, M.K.; Laborderie, J.; Lau, D.H.; Kuklik, P.; Shipp, N.J.; et al. Outcomes of long-standing persistent atrial fibrillation ablation: A systematic review. Heart Rhythm 2010, 7, 835–846. [Google Scholar] [CrossRef]
- Ouyang, F.; Tilz, R.; Chun, J.; Schmidt, B.; Wissner, E.; Zerm, T.; et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: Lessons from a 5-year follow-up. Circulation 2010, 122, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Cappato, R.; Calkins, H.; Chen, S.A.; Davies, W.; Iesaka, Y.; Kalman, J.; et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005, 111, 1100–1105. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.A.; Davies, W.; Iesaka, Y.; Kalman, J.; et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010, 3, 32–38. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.A.; Davies, W.; Iesaka, Y.; Kalman, J.; et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009, 53, 1798–1803. [Google Scholar] [CrossRef]
- Gupta, A.; Perera, T.; Ganesan, A.; Sullivan, T.; Lau, D.H.; Roberts-Thomson, K.C.; et al. Complication of catheter ablation of atrial fibrillation: A systematic review. Circ Arrhythm Electrophysiol. 2013, 6, 1082–1088. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Haegeli, L.M.; Brandes, A.; Heidbuchel, H.; Aliot, E.; Kautzner, J.; et al. Rationale and current perspective for early rhythm control therapy in atrial fibrillation. Europace 2011, 13, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Lip, G.Y.; Van Gelder, I.C.; Bax, J.; Hylek, E.; Kaab, S.; et al. Comprehensive risk reduction in patients with atrial fibrillation: Emerging diagnostic and therapeutic options—A report from the 3rd atrial fibrillation competence network/european heart rhythm association consensus conference. Europace 2012, 14, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Breithardt, G.; Aliot, E.; Khatib, S.A.; Apostolakis, S.; Auricchio, A.; et al. Personalized management of atrial fibrillation: Proceedings from the fourth atrial fibrillation competence network/european heart rhythm association consensus conference. Europace 2013. [Google Scholar] [CrossRef]
- Haegeli, L.M.; Duru, F.; Lockwood, E.E.; Luscher, T.F.; Sterns, L.D.; Novak, P.G.; et al. Ablation of atrial fibrillation after the retirement age: Considerations on safety and outcome. J Interv Card Electrophysiol. 2010, 28, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Haegeli, L.M.; Duru, F. Atrial fibrillation in the aging heart: Pharmacological therapy and catheter ablation in the elderly. Future Cardiol. 2011, 7, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Haegeli, L.M.; Duru, F. Management of patients with atrial fibrillation: Specific considerations for the old age. Cardiol Res Pract. 2011, 2011, 854205. [Google Scholar] [CrossRef] [PubMed][Green Version]
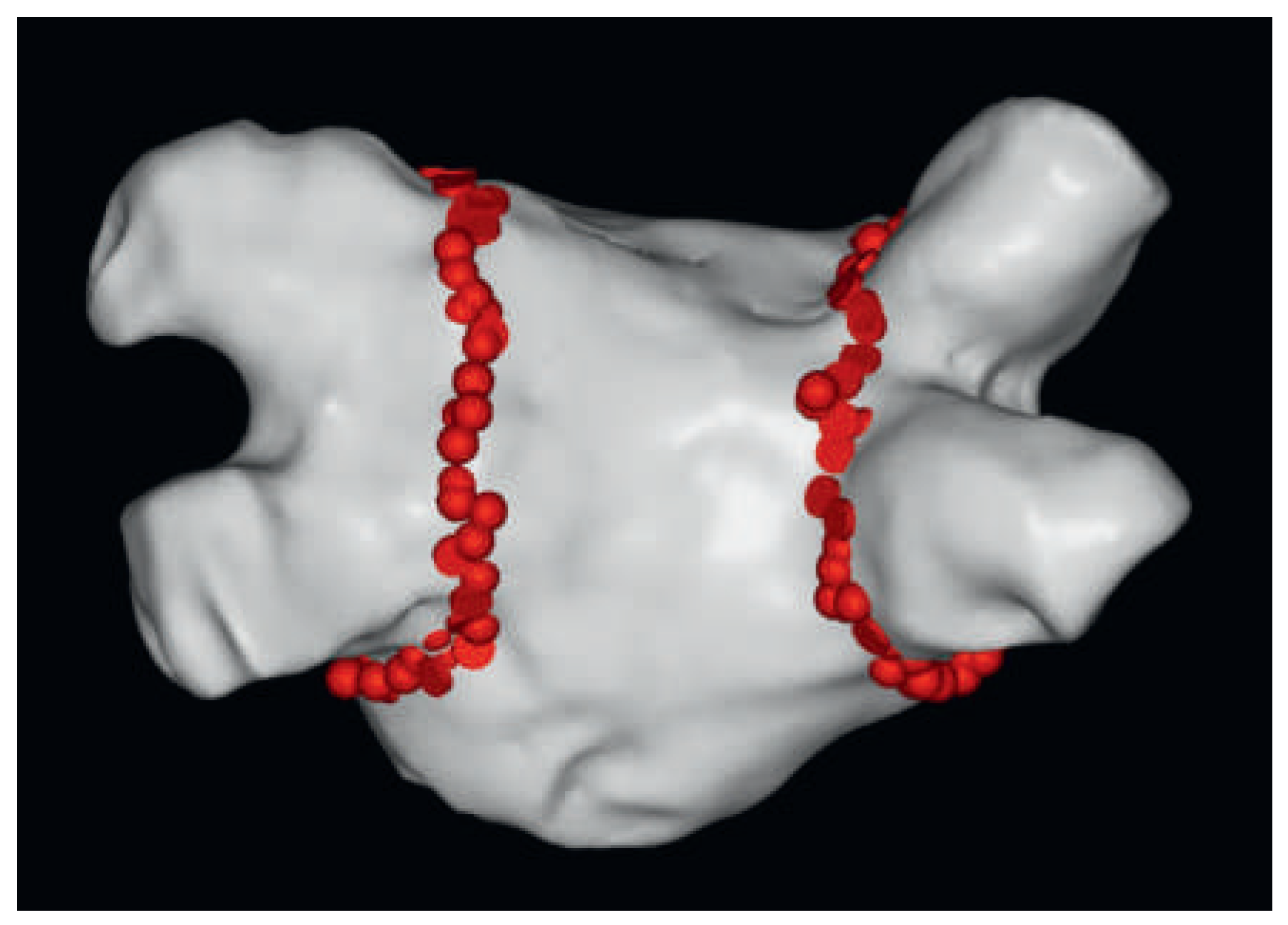
| Number of ablations | 275 | |
| Number of patients | 201 | |
| Ablations per patient | 1.37 | |
| Age (years) | 62 ± 9.09 | |
| Male (%) | 75 | |
| Left ventricular ejection fraction (EF) | 58 ± 8.06 | |
| Left atrium diameter (mm) | 44 ± 6.84 | |
| Comorbidities | ||
| Hypertension | 134 | 49% |
| Coronary artery disease | 35 | 13% |
| Diabetes mellitus | 23 | 8% |
| TIA/CVA | 28 | 10% |
| Heart failure | 10 | 4% |
| Type of AF | ||
| Paroxysmal AF | 125 | 62% |
| Persistent AF | 76 | 38% |
| CHA2DS2-VASc-Score | ||
| Patients with 0 points | 70 | 25% |
| Patients with 1 point | 63 | 23% |
| Patients with 2 points | 60 | 22% |
| Patients with 3 points | 46 | 17% |
| Patients with 4 points | 25 | 9% |
| Patients with 5 points | 10 | 4% |
| Patients with 6 points | 1 | 0.4% |
| Antiarrhythmic drugs before ablation | ||
| No antiarrhythmic drugs | 107 | 39% |
| With antiarrhythmic drugs | 168 | 61% |
| Amiodarone | 67 | 24% |
| Dronedarone | 48 | 17% |
| Sotalol | 3 | 1% |
| Flecainide | 34 | 12% |
| Propafenone | 14 | 5% |
| Digoxin | 9 | 3% |
| Paroxysmal AF before ablation | 125 | |
| 2-year follow-up available | 54 | 100% |
| SR | 38 | 70.4% |
| Paroxysmal AF | 15 | 27.8% |
| Persistent AF | 1 | 1.8% |
| Permanent AF | 0 | 0% |
| Persistent AF before ablation | 76 | |
| 2-year follow-up available | 22 | 100% |
| SR | 14 | 63.6% |
| Paroxysmal AF | 5 | 22.7% |
| Persistent AF | 2 | 9.1% |
| Permanent AF | 1 | 4.6% |
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haegeli, L.M.; Jud, F.; On, C.J.; Gstrein, C.; Saguner, A.M.; Steffel, J.; Benussi, S.; Krasniqi, N.; Brunckhorst, C.; Lüscher, T.F.; et al. Catheter Ablation for Atrial Fibrillation in a Real-World Setting. Cardiovasc. Med. 2015, 18, 319. https://doi.org/10.4414/cvm.2015.00356
Haegeli LM, Jud F, On CJ, Gstrein C, Saguner AM, Steffel J, Benussi S, Krasniqi N, Brunckhorst C, Lüscher TF, et al. Catheter Ablation for Atrial Fibrillation in a Real-World Setting. Cardiovascular Medicine. 2015; 18(11):319. https://doi.org/10.4414/cvm.2015.00356
Chicago/Turabian StyleHaegeli, Laurent M., Fabian Jud, Chol Jun On, Christine Gstrein, Ardan M. Saguner, Jan Steffel, Stefano Benussi, Nazmi Krasniqi, Corinna Brunckhorst, Thomas F. Lüscher, and et al. 2015. "Catheter Ablation for Atrial Fibrillation in a Real-World Setting" Cardiovascular Medicine 18, no. 11: 319. https://doi.org/10.4414/cvm.2015.00356
APA StyleHaegeli, L. M., Jud, F., On, C. J., Gstrein, C., Saguner, A. M., Steffel, J., Benussi, S., Krasniqi, N., Brunckhorst, C., Lüscher, T. F., Wolber, T., & Duru, F. (2015). Catheter Ablation for Atrial Fibrillation in a Real-World Setting. Cardiovascular Medicine, 18(11), 319. https://doi.org/10.4414/cvm.2015.00356




