Background
Diabetes mellitus increases the risk of cardiovascular disease [
1,
2,
3]. In addition to the well-recognised microvascular and macrovascular complications, diabetes also appears to increase risk of congestive heart failure [
4,
5]. Among survivors of acute myocardial infarction (MI), diabetes increases the risk of heart failure and cardiovascular disease [
6]. These increased risks appear to be independent of progressive ventricular enlargement [
5,
7].
The Healing and Early Afterload Reducing Therapy (HEART) study was a randomised, double-blind, placebo controlled trial of the haemodynamic effects of early versus delayed administration of ramipril after anterior Q-wave MI [
8]. The availability of data on diabetes, clinical presentation, echocardiographic changes, as well as heart failure and cardiovascular disease following MI afforded a unique opportunity to explore how diabetes might influence the relationship between clinical presentation of acute MI and outcomes.
Methods
Patients
The HEART trial enrolled 352 patients with Q-wave anterior MI to determine optimal timing of initiation of angiotensin-converting enzyme inhibitors. Patients were randomly assigned to receive one of three dosing regimens of ramipril: placebo for 14 days, followed by full-dose (10 mg) ramipril; lowdose (0.625 mg) ramipril for 90 days; or full-dose ramipril for 90 days. Patients with left bundle branch block were ineligible. Echocardiographic examinations were performed within the first 24 hours (baseline), at 14 days and 90 days after MI. Electrocardiograms performed within 24 hours (baseline) and prior to discharge (predischarge) after myocardial infarction were sent to the Data-Coordinating Centre for standardised and blinded assessment. The majority of patients (87%) received reperfusion therapy [
8].
Of the original 352 patients enrolled in HEART, 320 had interpretable baseline (median time from onset of symptoms = 3 hours) and predischarge (median time = day 7) electrocardiograms. Of these, 272 patients with analysable baseline and day 14 echocardiograms who also had either an acceptable day 90 echocardiogram (n = 268) or who died prior to the day 90 echocardiogram (n = 4) were included in the analysis.
Patients were classified as diabetic (n = 56, 21%) only if diabetes had been documented and therapy initiated prior to the index MI. Of these, 14 (25%) were treated with insulin, 31 (55%) with oral hypoglycaemic agents, and 11 (20%) with diet alone.
Echocardiographic analysis
Echocardiographic measurements were made on a Nova Microsonics workstation (Mahwah, New Jersey) as previously described [
8]. Endocardial borders from end-diastolic and end-systolic frames were digitised manually, and left ventricular volumes were calculated using the modified biplane Simpson’s rule to obtain left ventricular ejection fraction (EF). The total akinetic and dyskinetic segment length was assessed by manually tracing the akinetic or dyskinetic segment and was expressed as a percentage of endocardial perimeter. The reproducibility of the echocardiographic measurements had been previously reported [
8].
Statistical analyses
Descriptive statistics (percentages for binomial variables, mean ± standard deviation [SD] for variables with approximately normal distributions, 25th to 75th percentile for variables with skewed distribution) were used for clinical, electrocardiographic and echocardiographic characteristics in diabetics and non-diabetics. Comparisons between diabetic and non-diabetic groups were performed using χ2 tests for differences in the proportions of categorical variables, Student’s t-test for continuous variables, and two-sample Wilcoxon rank sum test for ordinal variables and variables that were not normally distributed.
Outcome measures included hospitalisation for heart failure, non-fatal recurrent MI, or cardiovascular disease events, a composite of death, hospitalisation for heart failure, or MI. Patients were qualified as being hospitalised for heart failure, if heart failure was deemed the primary cause for the hospitalisation and for which additional medication was administered, i.e., diuretic, intravenous or oral nitrates, intravenous inotropic agents.
All endpoints were ascertained by the site investigators and filled out on a case report form. The frequencies of outcomes in the two groups were compared using logistic regression analysis. The associations between each outcome variable and diabetes were assessed with multiple logistic regression analysis, including factors in the model that had statistically significant associations (at p <0.05) with outcomes (i.e., baseline left ventricular EF, baseline akinetic/dyskinetic segment length, hypertension, maximal creatine kinase, age, and prior MI). The continuous variables satisfied the assumption of linearity. This assumption was checked by sub-dividing each continuous variable into four sub-groups corresponding to each quartile of the distribution, examining the beta-coefficients of the logistic regression within each group, and determining that these were similar across the range.
A two-sided p-value of less than 0.05 was considered to indicate statistical significance in all analyses. All statistical analyses were performed with Stata® 7 (Stata Corporation, College Station, TX).
Results
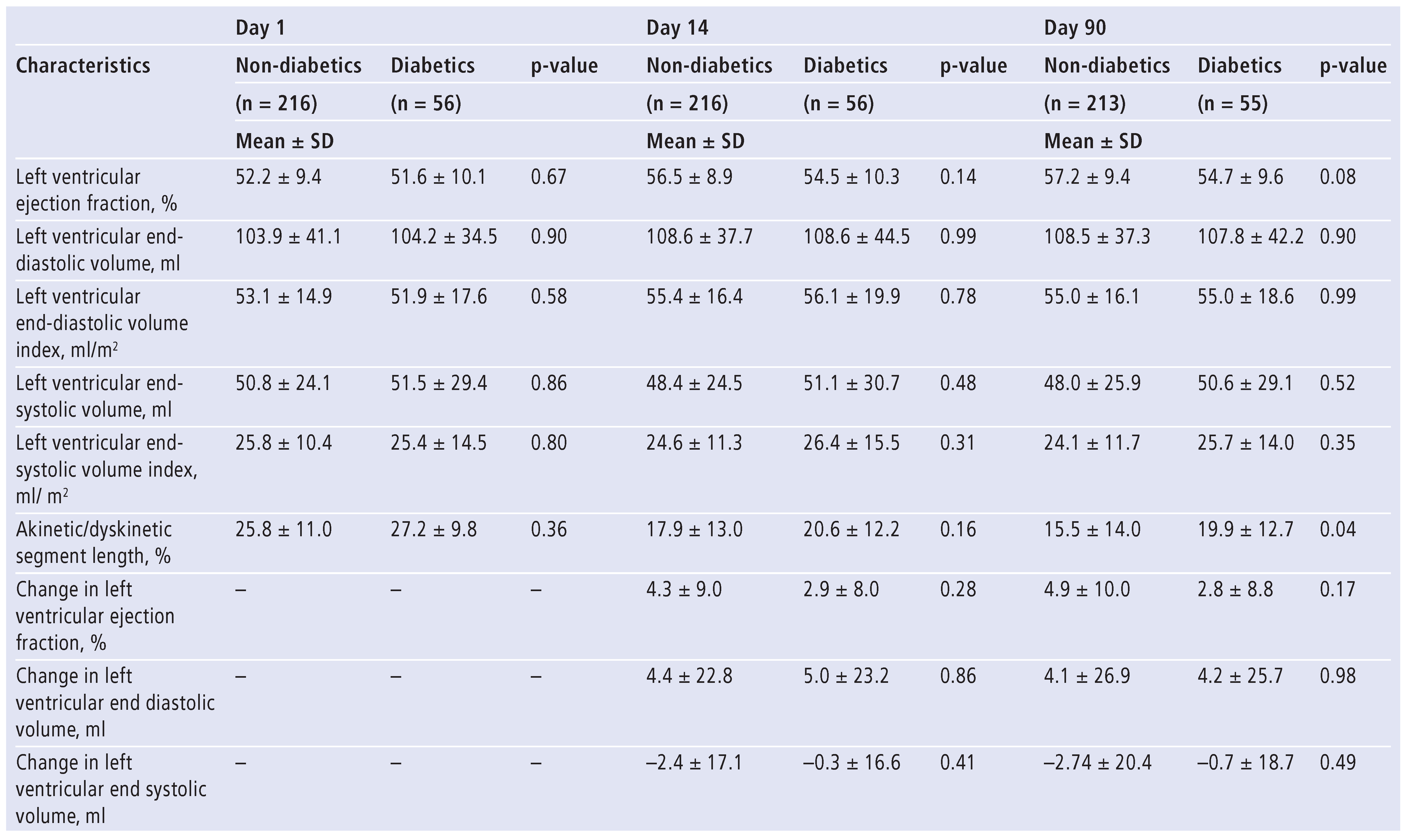
Baseline characteristics in diabetic and non-diabetic patients are presented in
Table 1. At presentation, diabetic and non-diabetic patients were similar with respect to clinical features, baseline medication, left ventricular volumes and EF, maximal creatine kinase levels and akinetic/dyskinetic segment length (
Table 1 and
Table 2). Nevertheless, a higher proportion of diabetics than non-diabetics presented with Killip class >1 (34% versus 19%, p = 0.01). Two weeks after MI, diabetic and non-diabetic patients were similar with respect to left ventricular EF, end-systolic and end-diastolic volume, and akinetic/dyskinetic segment length (
Table 2). Similarly, at day 90, left ventricular volumes were similar in the two groups although diabetic patients demonstrated greater akinetic/dyskinetic segment length and slightly, though not significantly, lower EF (
Table 2). Complete recovery of ventricular function (EF > 55% and absence of regional wall motion abnormalities) was seen in 25% of non-diabetics and 20% of diabetics (p = 0.37). In a subset of patients (non-diabetics = 104, diabetics = 27), echocardiographic parameters of diastolic function were similar in both groups at the three time points (
Table 3).
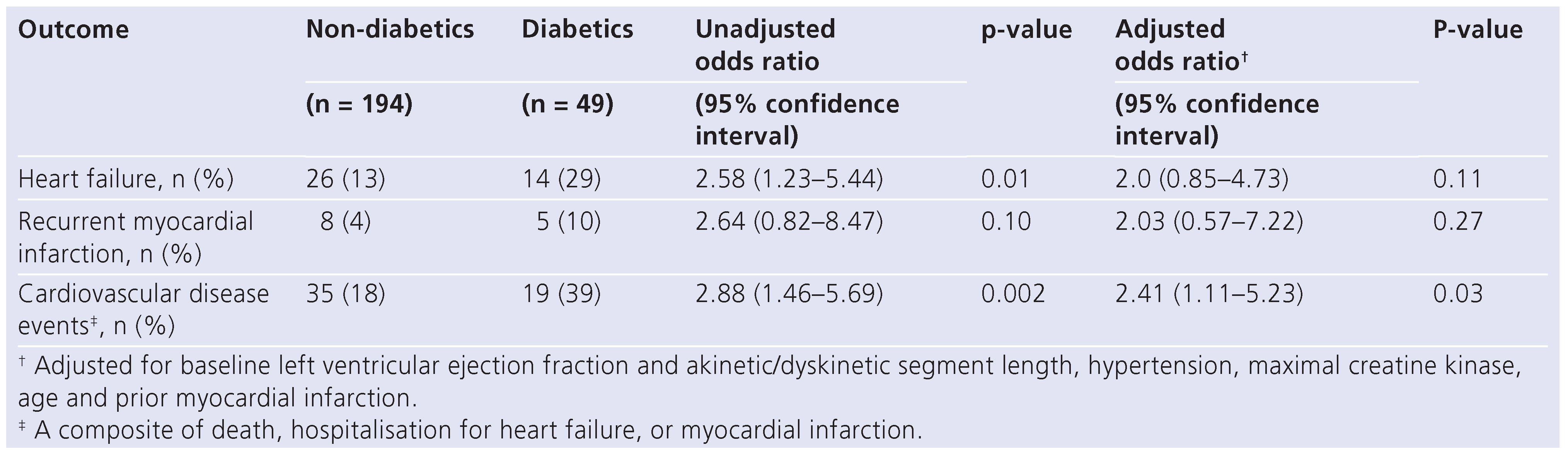
The one-year follow-up status was available in 243 (89%) patients. In the year following infarction, diabetics had a higher incidence of either hospitalisation for heart failure or cardiovascular disease events (
Table 4). The difference in total cardiovascular disease events was primarily due to increased congestive heart failure and remained significant even after adjusting for baseline left ventricular EF and akinetic/dyskinetic segment length, hypertension, maximal creatine kinase, age, and prior MI (
Table 4).
Discussion
The results of this study demonstrated that diabetics are at increased risk of developing congestive heart failure even after myocardial infarction with mild left ventricular dysfunction or preserved ejection fraction. In this anterior MI population, in which the majority of patients received reperfusion therapy, the increased risk of heart failure in diabetic patients was not attributable to differences in initial measures of infarct size, ventricular function, or subsequent ventricular remodelling.
Several studies have suggested that diabetics, with or without previous cardiovascular disease, have up to five-fold increased risk of heart failure [
4,
5,
9,
10,
11,
12]. While diabetes accelerates and enhances the atherosclerotic process [
13], as reflected by the greater extent and severity of coronary artery disease in diabetic patients [
14], diabetics do not appear to have more extensive infarctions than non-diabetic patients [
15,
16]. In the present study, infarcts were of mild to moderate extent (based on akinetic/dyskinetic segment length, EF, and maximal creatine kinase) and the infarct size was comparable between diabetics and non-diabetics. While left ventricular EF was relatively high in this post-MI population, diabetics still demonstrated a significantly higher risk of developing heart failure or total cardiovascular disease events. These data are compatible with the hypothesis that factors other than infarct size or systolic function must account for the increased incidence of heart failure observed in diabetics.
Left ventricular remodelling, occurring in some patients after MI, is a continuous process influenced by a variety of factors, including infarct size [
17], coronary reperfusion [
18], and angiotensin-converting enzyme inhibitor therapy [
19,
20], and has been thought to represent an important component in the progression to heart failure [
21]. In a previous report from patients enrolled in the Survival and Ventricular Enlargement (SAVE) study, diabetes was not associated with increased remodelling in patients with left ventricular dysfunction following MI despite a marked increase in heart failure [
5]. These observations, however, were in a selected population of patients with large infarcts and left ventricular dysfunction (EF ≤40% per protocol), and therefore at high risk for developing ventricular remodeling and heart failure. In fact, the present study consisted of patients with mild to moderate infarcts (mean baseline EF = 52%), in which, by day 90, complete recovery of ventricular function occurred in 22% of the population and heart failure developed in a minority (17%) [
17]. Yet in this population, diabetes likewise increased the risk of heart failure and total cardiovascular disease independently of the risk of remodelling.
The observation that patients with diabetes presented with a higher Killip class suggests that diabetes may influence myocardial function very early in the infarct process. Several lines of evidence support the hypothesis [
22] that in the diabetic heart altered myocardial energy metabolism may adversely affect myocardial function [
23]. Still, we did not observe significant differences between groups with respect to systolic function, raising the possibility that differences in diastolic function might account for the increased heart failure risk in the diabetic patient although we do not have specific data to support this hypothesis. In patients with well controlled and uncomplicated type 2 diabetes, there was a strong association between impaired left ventricular diastolic function and reduced myocardial high-energy phosphate metabolism [
24]. Although diastolic dysfunction is a known feature of diabetic cardiomyopathy and may contribute to the pathogenesis of heart failure [
25,
26,
27] there is no strong evidence supporting a causal relationship between abnormal diastolic function and heart failure in diabetic patients.
Study limitations
In the HEART study the diagnosis of diabetes was based on information requested of the patients at screening evaluation on the awareness of the disease and/or presence of anti-diabetic treatments – insulin, oral hypoglycaemic agents, or diet alone. Since, at that time, we did not ask for glucose test, glucose tolerance test, or glycated haemoglobin (HbA
1c) at baseline, we could neither base the diagnosis of diabetes on these clinical markers nor detect new cases of diabetes. Therefore, in this study patients with diabetes included only patients with previously known diagnosis of diabetes and did not include patients with newly diagnosed diabetes which may constitute about 4% of patients with myocardial infarction [
28]. This might imply that we underestimated the proportion of patients with diabetes.
The HEART study population consisted of patients with anterior myocardial infarction of whom only 25% underwent primary percutaneous coronary intervention (PCI). Therefore, the results from this study could not be translated to today’s clinical practice, as nowadays the majority of patients are treated with primary PCI.
Due to the fact that the majority of patients did not undergo primary PCI, we also lack angiographic data for our study population and therefore we cannot exclude that at baseline diabetic patients had more extensive coronary disease, although we do not think this could have influenced the main results of the present study.
Conclusions
In a well characterised population of patients with anterior MI and mild residual left ventricular dysfunction or preserved ejection fraction, diabetes is associated with worse functional class and a greater risk of developing congestive heart failure in the following year. This increased risk of heart failure in diabetic patients appears to be independent of infarct size, systolic function and subsequent left ventricular remodelling. Their higher Killip class contributes to the formulation of the hypothesis that diastolic dysfunction may have aetiologic importance in the observed higher risk of heart failure among diabetic patients who survive myocardial infarction.
Funding/potential competing interests
Dr. Pfeffer reports having served as consultant to Aastrom, Abbott Vascular, Amgen, Bristol-Myers Squibb, Cerenis, Concert, Keryx, Medtronic, Merck, Novartis, Roche, Servier, Teva and having received grant support from Amgen, Celladon, Novartis, and Sanofi-Aventis. The Brigham and Women’s Hospital has patents for the use of inhibitors of the renin-angiotensin system in selected survivors of MI with Novartis Pharmaceuticals. Dr. Pfeffer is a co-inventor. His share of the licensing agreement is irrevocably transferred to charity. Dr. Hennekens serves as independent scientist in an advisory role to investigators and sponsors as Chair or Member of Data and Safety Monitoring Boards for Amgen, Bayer, Bristol Myers-Squibb, British Heart Foundation, Cadila, Canadian Institutes of Health Research, Genzyme, Lilly, Sunovion and the Wellcome Foundation, and as an independent scientist in an advisory role to Pfizer, the United States (U.S.) Food and Drug Administration, and UpToDate.
References
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- Lenzen, M.; Ryden, L.; Ohrvik, J.; Bartnik, M.; Malmberg, K.; Scholte Op Reimer, W.; et al. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1–year outcome in patients with coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. Eur Heart J. 2006, 27, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Lehto, S.; Ronnemaa, T.; Pyorala, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998, 339, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; St John Sutton, M.; Lamas, G.A.; Plappert, T.; Rouleau, J.L.; Skali, H.; et al. Ventricular remodeling does not accompany the development of heart failure in diabetic patients after myocardial infarction. Circulation 2002, 106, 1251–1255. [Google Scholar] [CrossRef]
- Shah, R.V.; Holmes, D.; Anderson, M.; Wang, T.Y.; Kontos, M.C.; Wiviott, S.D.; et al. Risk of heart failure complication during hospitalization for acute myocardial infarction in a contemporary population: insights from the National Cardiovascular Data ACTION Registry. Circ Heart Fail. 2012, 5, 693–702. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Janus, M.; Prech, M.; Grygier, M.; Pyda, M.; Olasinska-Wisniewska, A.; et al. Relations of diabetes mellitus, microvascular reperfusion and left ventricular remodelling in patients with acute myocardial infarction treated with primary coronary intervention. Kardiol Pol. 2014, 72, 20–26. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Greaves, S.C.; Arnold, J.M.; Glynn, R.J.; LaMotte, F.S.; Lee, R.T.; et al. Early versus delayed angiotensin-converting enzyme inhibition therapy in acute myocardial infarction. The healing and early afterload reducing therapy trial. Circulation 1997, 95, 2643–2651. [Google Scholar] [CrossRef]
- Nichols, G.A.; Hillier, T.A.; Erbey, J.R.; Brown, J.B. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001, 24, 1614–1619. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001, 161, 996–1002. [Google Scholar] [CrossRef]
- Gustafsson, I.; Torp-Pedersen, C.; Kober, L.; Gustafsson, F.; Hildebrandt, P. Effect of the angiotensin-converting enzyme inhibitor trandolapril on mortality and morbidity in diabetic patients with left ventricular dysfunction after acute myocardial infarction. Trace Study Group. J Am Coll Cardiol. 1999, 34, 83–89. [Google Scholar] [CrossRef]
- Aronson, D.; Rayfield, E.J.; Chesebro, J.H. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997, 126, 296–306. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef]
- Woodfield, S.L.; Lundergan, C.F.; Reiner, J.S.; Greenhouse, S.W.; Thompson, M.A.; Rohrbeck, S.C.; et al. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. 1996, 28, 1661–1669. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Spadaro, J.J.; Schechtman, K.; Roberts, R.; Geltman, E.M.; Sobel, B.E. Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J. 1984, 108, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Gwilt, D.J.; Petri, M.; Lewis, P.W.; Nattrass, M.; Pentecost, B.L. Myocardial infarct size and mortality in diabetic patients. Br Heart J. 1985, 54, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Glynn, R.J.; Greaves, S.; Ajani, U.; Rouleau, J.L.; Menapace, F.; et al. Recovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early afterload reducing therapy study. Ann Intern Med. 2001, 134, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Choo, H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation 1987, 75, 299–306. [Google Scholar] [CrossRef]
- St John Sutton, M.; Pfeffer, M.A.; Plappert, T.; Rouleau, J.L.; Moye, L.A.; Dagenais, G.R.; et al. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994, 89, 68–75. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Lamas, G.A.; Vaughan, D.E.; Parisi, A.F.; Braunwald, E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988, 319, 80–86. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef]
- Young, M.E.; McNulty, P.; Taegtmeyer, H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation 2002, 105, 1861–1870. [Google Scholar] [CrossRef]
- Lopaschuk, G.D. Abnormal mechanical function in diabetes: relationship to altered myocardial carbohydrate/lipid metabolism. Coron Artery Dis. 1996, 7, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Diamant, M.; Lamb, H.J.; Groeneveld, Y.; Endert, E.L.; Smit, J.W.; Bax, J.J.; et al. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003, 42, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Solang, L.; Malmberg, K.; Ryden, L. Diabetes mellitus and congestive heart failure. Further knowledge needed. Eur Heart J. 1999, 20, 789–95. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Klein, L.; Gheorghiade, M.; Bonow, R.O. New insights into diastolic heart failure: role of diabetes mellitus. Am J Med. 2004, 116 (Suppl 5A), 64S–75S. [Google Scholar] [CrossRef]
- Galderisi, M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol. 2006, 48, 1548–1551. [Google Scholar] [CrossRef]
- Aguilar, D.; Solomon, S.D.; Kober, L.; Rouleau, J.L.; Skali, H.; McMurray, J.J.; et al. Newly diagnosed and previously known diabetes mellitus and 1–year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation 2004, 110, 1572–1578. [Google Scholar] [CrossRef]
Table 1.
Baseline characteristics of non-diabetics (n = 216, 79%) and diabetics (n = 56, 21%).
Table 1.
Baseline characteristics of non-diabetics (n = 216, 79%) and diabetics (n = 56, 21%).
| Characteristics | Non-diabetics | Diabetics | p-value |
|---|
| | (n = 216) | (n = 56) | |
| | Number (%) or mean ± SD | | |
| Age, yr (SD) | 59.6 ± 13.1 | 60.8 ± 10.9 | 0.50 |
| Male, n (%) | 172 (80) | 40 (71) | 0.19 |
| Body mass index, kg/m2 | 27.0 ± 4.3 | 29.3 ± 5.5 | 0.003 |
| Body surface area, m2 | 1.9 ± 0.3 | 1.9 ± 0.3 | 0.96 |
| Killip class >1, n (%) | 40 (19) | 19 (34) | 0.01 |
| Systolic blood pressure day 1, mm Hg | 118.8 ± 15.1 | 124.0 ± 17.1 | 0.01 |
| Diastolic blood pressure day 1, mm Hg | 71.2 ± 10.3 | 71.5 ± 12.7 | 0.8 |
| Maximal creatine kinase, mU/ml | 2484 ± 2012 | 2593 ± 1887 | 0.71 |
| Creatinine, mg/dl | 1.1 ± 0.24 | 1.0 ± 0.23 | 0.29 |
| Total serum cholesterol, mg/dl | 209.4 ± 49.1 | 212.4 ± 48.1 | 0.71 |
| Hypertension, n (%) | 83 (39) | 28 (50) | 0.13 |
| Previous myocardial infarction, n (%) | 30 (14) | 13 (23) | 0.09 |
| Previous angina, n (%) | 112 (40) | 29 (40) | 0.98 |
| Duration of previous angina, weeks | 15.8 ± 90 | 7.4 ± 10.3 | 0.62 |
| Reperfusion therapy, n (%) | 192 (89) | 45 (80) | 0.09 |
| Thrombolytic | 142 (74) | 34 (76) | 0.11 |
| PTCA | 50 (26) | 11 (24) | 0.57 |
| PTCA left anterior descendent, n (%) | 71 (90) | 18 (95) | 0.51 |
| Aspirin, n (%) | 202 (93) | 49 (87) | 0.13 |
| Diuretics, n (%) | 39 (18) | 14 (25) | 0.20 |
| Digitalis, n (%) | 7 (3) | 2 (4) | 0.90 |
| Beta-blockers, n (%) | 158 (73) | 36 (64) | 0.19 |
| Treatment group, n (%) | | | |
| Placebo, then full-dose ramipril | 75 (35) | 12 (21) | |
| Low-dose ramipril | 71 (33) | 19 (34) | 0.11 |
| Full-dose ramipril | 70 (32) | 25 (45) | |
| PTCA = percutaneious transluminal coronary angioplasty |
Table 2.
Echocardiographic characteristics at day 1, day 14, and day 90 of non-diabetics (n = 216) and diabetics (n = 56).
Table 2.
Echocardiographic characteristics at day 1, day 14, and day 90 of non-diabetics (n = 216) and diabetics (n = 56).
Table 3.
Echocardiographic assessment of diastolic function at day 1, day 14, and day 90 of non-diabetics (n = 104) and diabetics (n = 27).
Table 3.
Echocardiographic assessment of diastolic function at day 1, day 14, and day 90 of non-diabetics (n = 104) and diabetics (n = 27).
Table 4.
Clinical events of non-diabetics (n = 194) and diabetics (n = 49) in the first year following myocardial infarction.
Table 4.
Clinical events of non-diabetics (n = 194) and diabetics (n = 49) in the first year following myocardial infarction.
© 2014 by the author. Attribution-Non-Commercial-NoDerivatives 4.0.