Case
A 72-year-old male patient, with severe “low-flow-lowgradient” aortic stenosis (left ventricular ejection fraction (LVEF): 20%, mean pressure gradient: 26 mm Hg, valve orifice area: 0.7 cm2) was referred to our hospital for further therapeutic evaluation. Clinical symptoms at time of admission were those of congestive heart failure with dyspnoea NYHA III–IV. Only three months previously this patient had undergone balloon valvuloplasty (BAV) of the stenotic aortic valve. To improve his severely reduced clinical status, a second BAV was performed one week prior to surgery resulting in a mere decrease of his mean aortic pressure gradient from 15 mm Hg to 9 mm Hg. Unfortunately, there was no significant clinical improvement and indication for surgical or interventional treatment was given. Preoperative work-up was extensive and showed concomitant arterial hypertension, dyslipidaemia, diabetes mellitus type 2, obesity (BMI 38 kg/m2), a history of smoking (75 PY), permanent atrial fibrillation as well as a minimal-change glomerulonephritis with renal insufficiency (stage 3–4). Cumulative Euroscore II was 10.5%.
A CT scan was performed to evaluate aortic annular dimensions and the appropriateness for transcatheter aortic valve implantation (TAVI). However, this approach had to be rejected due to an annulus size of 34 × 27 mm (circumference of 94 mm), which would have required a larger valve than available at present. The CT scan also revealed a severely calcified ascending aorta (“porcelain aorta”), but only minor calcifications in the descending aorta. In this setting a conventional AVR was rejected because of the high risk associated with clamping of the aorta for cardioplegic arrest. Additionally, conventional AVR would have required the use of CPB, with the risk of exacerbation of the already compromised renal situation. After echocardiographic exclusion of a severe aortic valve insufficiency, which is an exclusion criterion for aortic valve bypass surgery (AVB), this treatment option was considered to be best for this patient.
The AVB consists of two components: a straight valve-containing conduit with a 23 mm porcine valve and an 18 mm angled left ventricular connector (Correx, Inc., Waltham, MA, USA). Conceptionally the AVB preempts the stenotic aortic valve by bypassing the blood-flow via left ventricular apex into the descending aorta.
Figure 1.
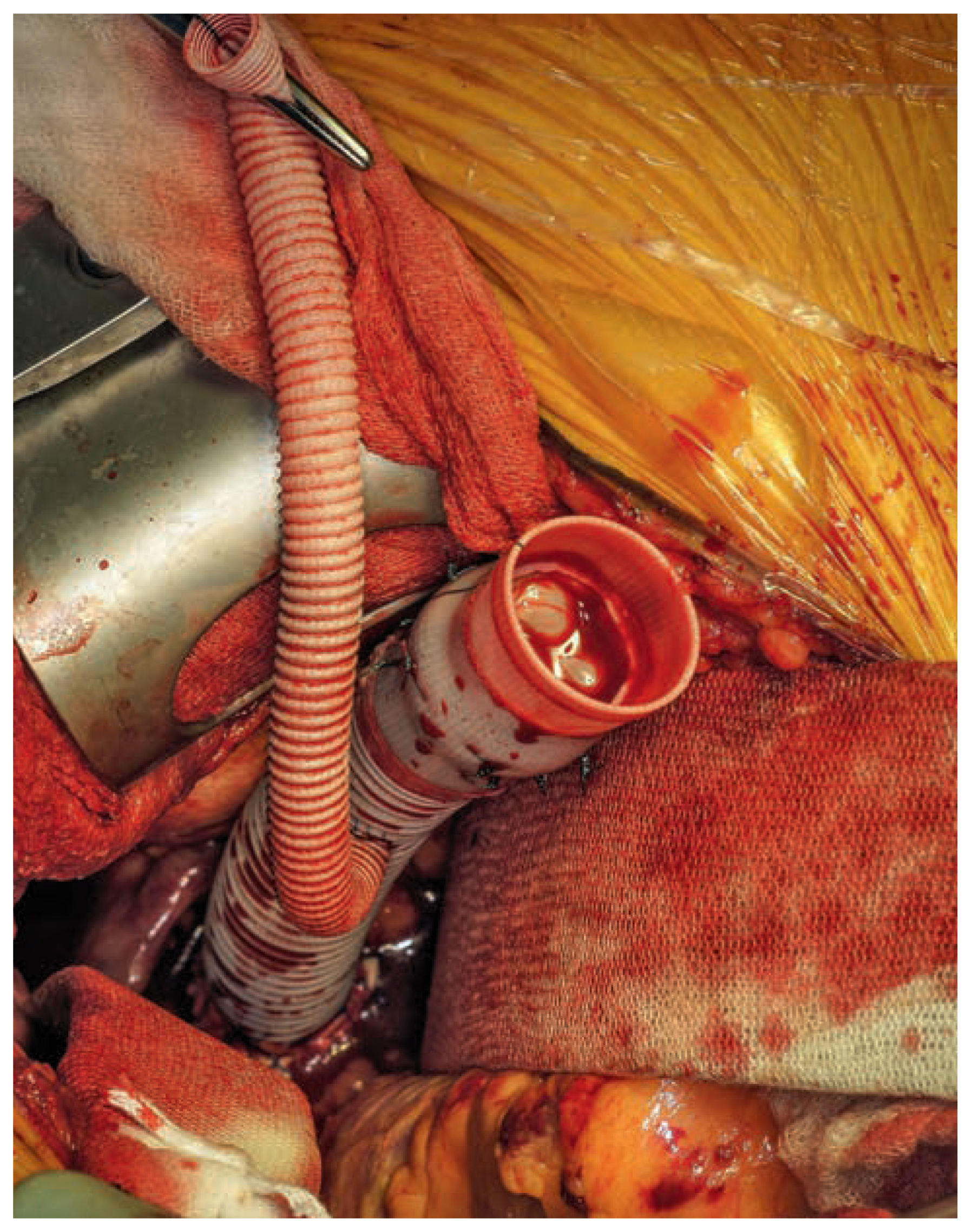
Valved conduit with the attached 8 mm Dacron® side-branch already grafted to the descending aorta.
Figure 1.
Valved conduit with the attached 8 mm Dacron® side-branch already grafted to the descending aorta.
Figure 2.
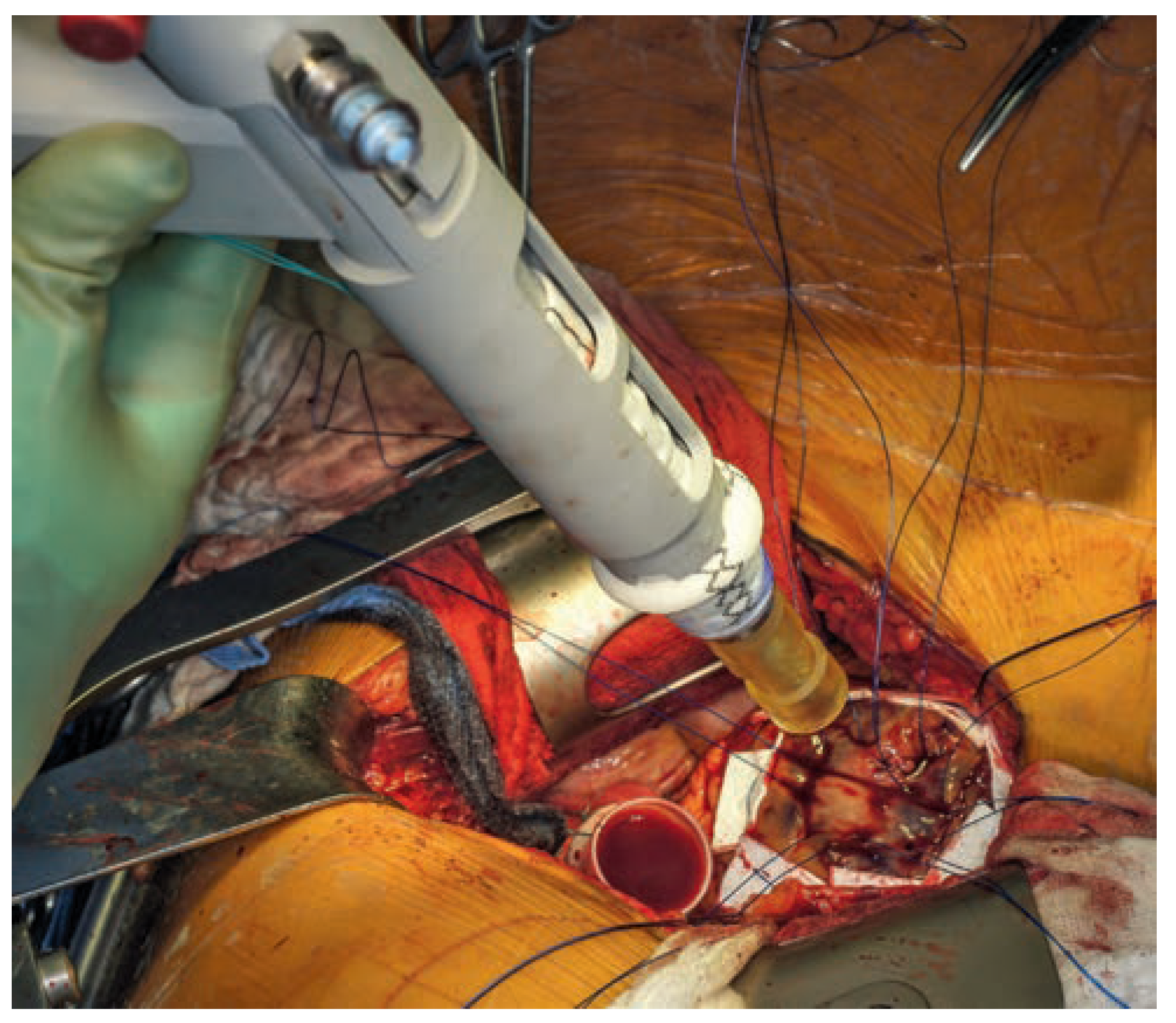
The gun-like applicator with mounted left ventricular connector and flushed balloon.
Figure 2.
The gun-like applicator with mounted left ventricular connector and flushed balloon.
The patient was placed in the right lateral decubitus position. CPB was on standby. A guide-wire was placed via the left femoral vein in the right atrium for possible venous access and an 8 mm vascular graft was attached to the valved conduit. This setting was supposed for controlled access for ECC in case of emergency. The operation was performed off-pump, through a left anterolateral thoracotomy with right-sided one-lung ventilation. After administering heparin, a side-biting clamp was placed on the descending aorta. After adjustment of length to prevent kinking, the valved conduit with the 8 mm vascular tube was end-to-side grafted to the descending aorta with pledgeted single prolene 3/0 sutures. Haemostasis was meticulously controlled.
Figure 3.
Pledgeted 2/0 prolene sutures for fixation of the LV-connector at the apex.
Figure 3.
Pledgeted 2/0 prolene sutures for fixation of the LV-connector at the apex.
Figure 4.
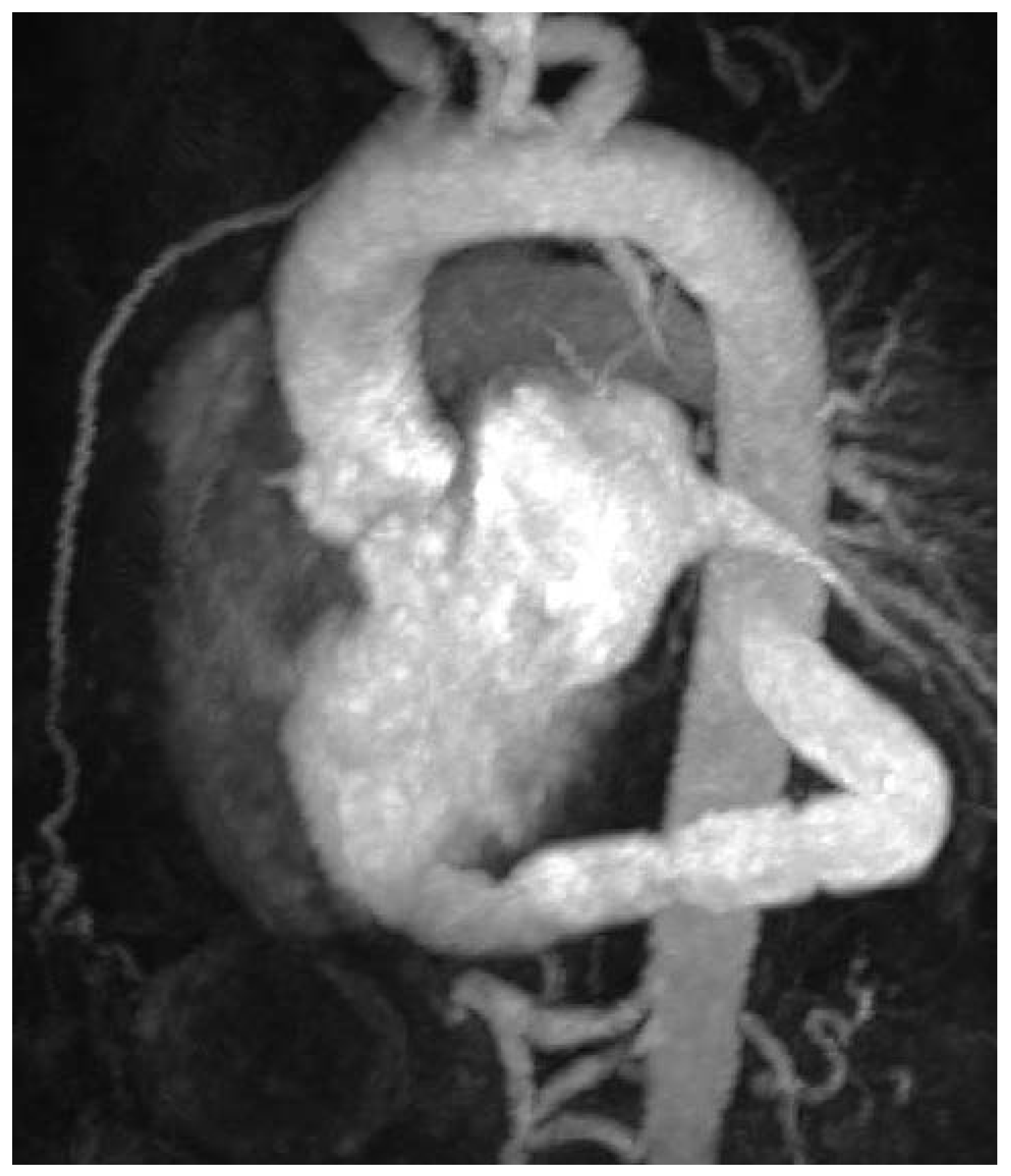
Postoperative MRI showing correct position of the AVB.
Figure 4.
Postoperative MRI showing correct position of the AVB.
After opening the pericardium, the insertion position of the 18 mm apical connector was marked lateral to the apex. Seven pledgeted prolene 2/0 sutures were passed circumferentially through the myocardium. By means of the automated coring device, the apical connector was placed off-pump in the ventricular wall and sutures carefully tied. The coring device was removed and both conduits connected. They were secured with prolene 4/0 sutures as well as umbilical tape. The deairing clamps were released and flow initiated. Haemostasis chest tubes were inserted and thoracotomy closed in usual fashion.
Despite preexisting atrial fibrillation, the patient stayed haemodynamically stable throughout the operation requiring only low doses of noradrenalin, adrenalin and fluid. Heparin was fully reversed with protamine. He was given one unit of red blood cell concentrate and 240 ml of cell-saver blood were transfused.
Intraoperative transoesophageal echocardiography confirmed the left ventricular EF of 22%. Positioning of the apical connector was as predicted with no interference with septal tissue or the subvalvular apparatus. Along the course of the conduit no stenosis or obstruction was detected.
The postoperative course was uneventful except for a delirium, which was medically treated. Augmentin® was given preemptively up to the tenth postoperative day to prevent wound infection.
A MRI scan of the heart was performed on day fourteen after surgery. Flow measurement demonstrated 55% of cardiac output passed via conduit. Cardiac output of 2.1 l/min was measured in the ascending aorta and of 2.6 l/min in the descending aorta, with a total of 4.7 l/min. Minimal flow was shown in the descending aorta distal to the subclavian artery and proximal to the conduit. Bioprosthetic regurgitation was excluded in the MRI.
The patient was discharged for routine cardiac rehabilitation with greatly improved health condition in NYHA class I–II.
Follow-up after three months revealed stable clinical conditions in a satisfied patient remaining in NYHA class I. He is again capable of undertaking normal daily activities and longer walks up to 5 km. Because of musculoskeletal degeneration due to longer immobilisation, he is still improving with physical therapy.
Discussion
With the rise of TAVI nowadays as well as extensive experience in conventional aortic valve surgery the vast majority of patients suffering from aortic stenosis can be safely treated. However, there is a cohort of patients that may benefit from this novel off-pump implantation of a valved apico-descending graft, the Correx® AVB [
1]. In the presented case the large size of the annulus prevented implantation of a TAVI and the porcelain ascending aorta as well as the impaired renal function prohibited the use of extracorporeal circulation for conventional aortic valve replacement.
The concept of apico-aortic conduits is long known. In the mid-1950’s Sarnoff et al. published his idea of treating aortic stenosis [
2]. However, the need for extracorporeal circulation and the lack of appropriate instrumentarium made the procedure highly sophisticated resulting in a low adoption of the technique amidst the surgical community. Hence, patients treated with this technique show good long-term results with improved quality of life [
3]. Vliek et al. have also shown that after aortic valve bypass surgery there is a longterm stability of left ventricular haemodynamics with no progression of native aortic valve stenosis. The flow distribution through the native aortic valve and the conduit can be predicted and additionally there is an increase in stroke volume and midterm preservation of LVEF [
4].
In 2012 the group of Gammie described a novel tool for facilitated implantation of an AVB without the need for extracorporeal circulation [
5]. This technique encompasses a gun-like applicator that not only cores the apex, but also secures the tissue plug, cares for haemostasis and implants the left ventricular connector.
With this method in mind, we appraised our patient to be an appropriate candidate for this treatment. Despite his severe obesity, the reduced ejection fraction as well as the long-standing persistent atrial fibrillation, the intraoperative course was uneventful. As was the postoperative history with only transient delirium resulting in discharge on postoperative day 15 and significant improvement of NYHA classification.
In conclusion we could show the effectiveness of this novel technique of off-pump AVB implantation in a patient otherwise not amenable for treating his severe aortic stenosis.