"Congenital" Chest Pain–Anomalous Origin of the Left Anterior Descending Coronary Artery from the Pulmonary Artery
Abstract
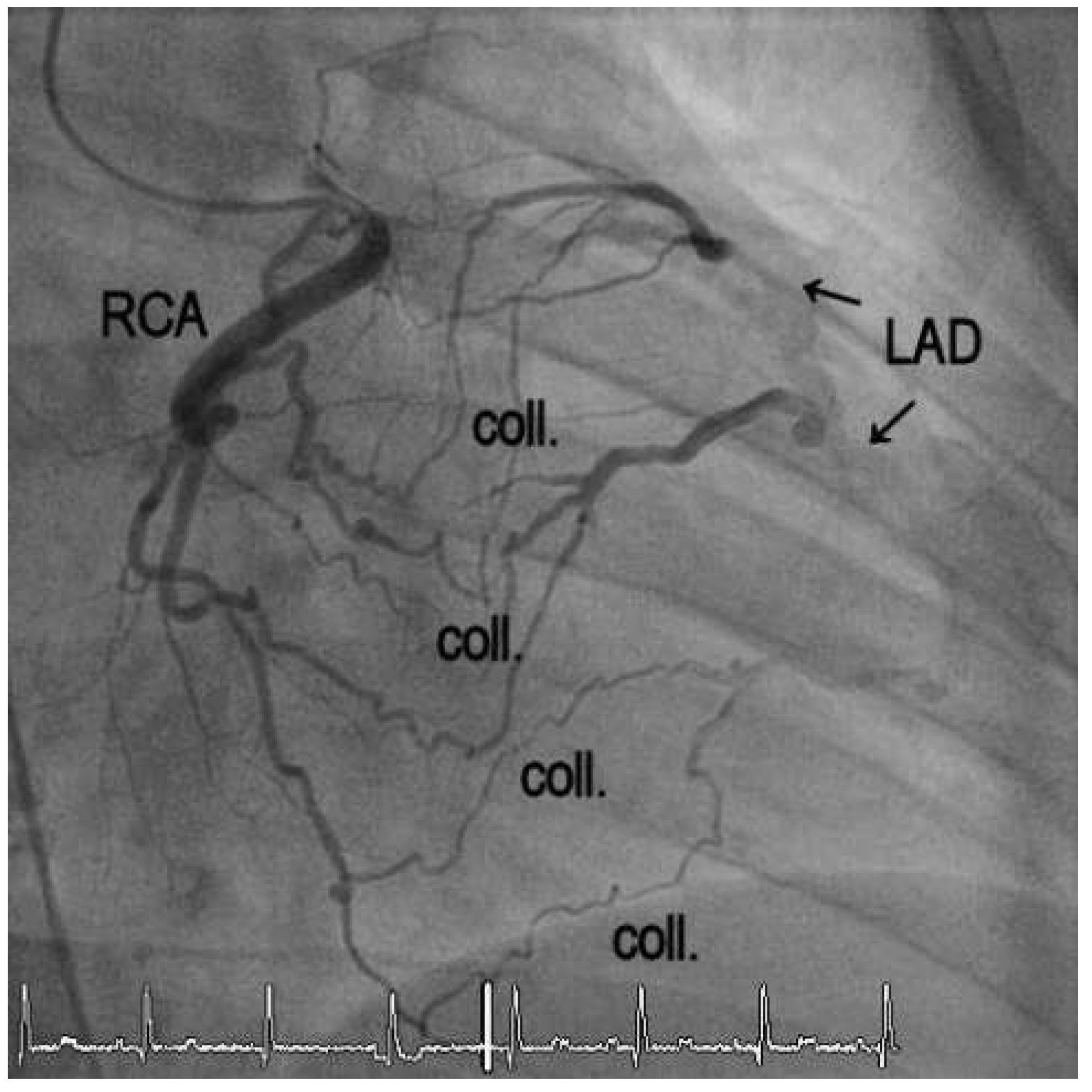
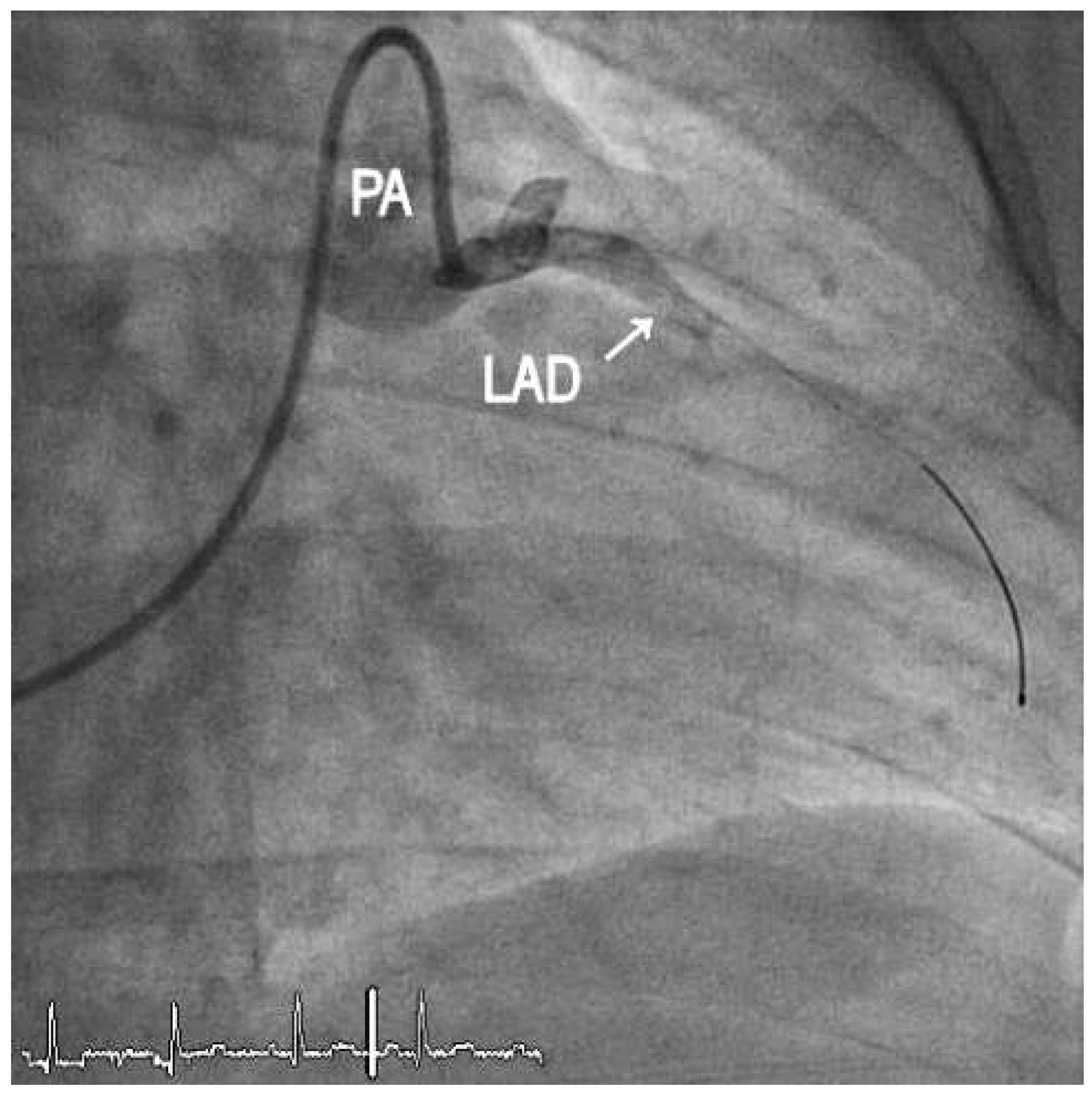
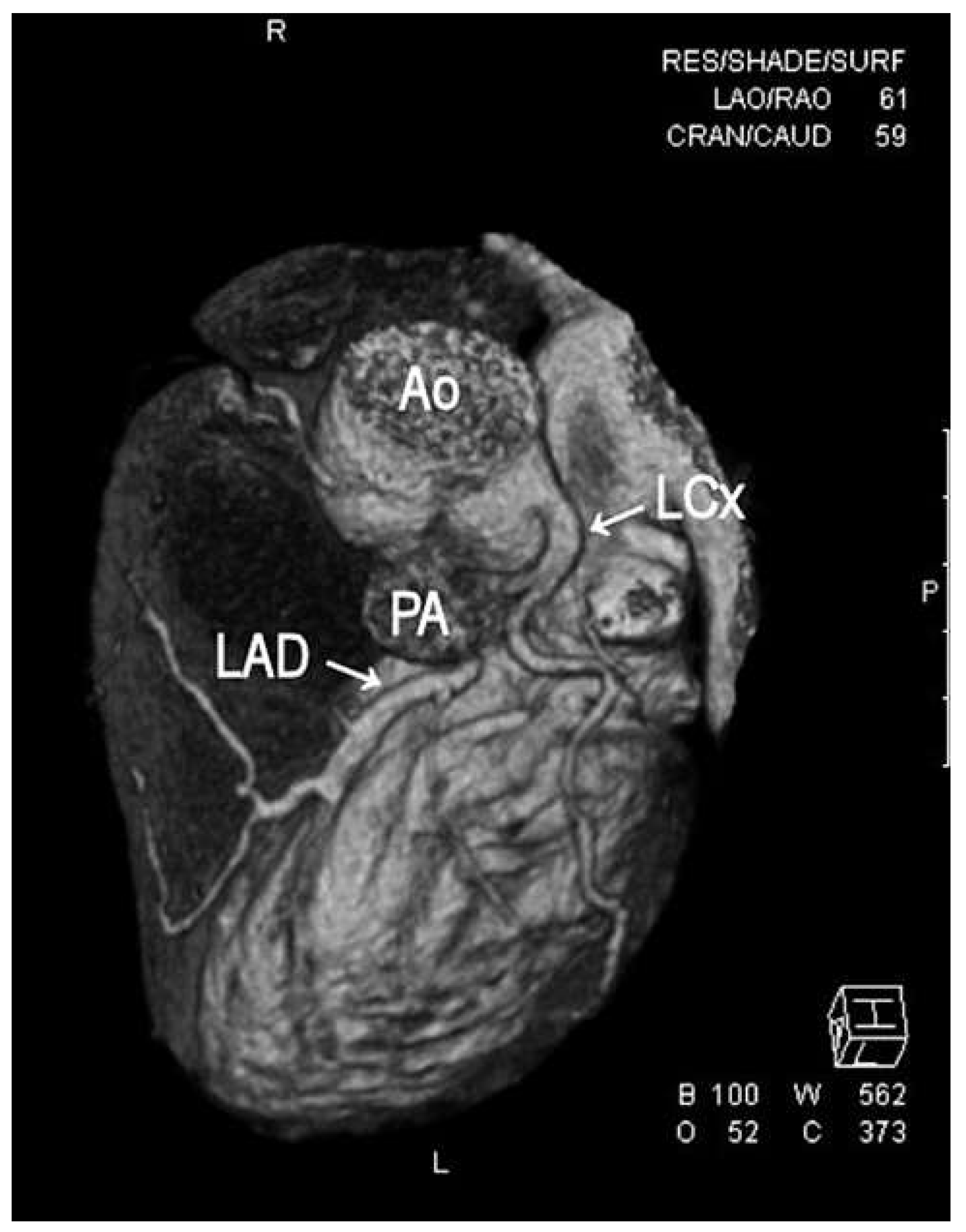
1. Case Report
2. Discussion
Funding
Conflicts of Interest
References
- Yamanaka, O.; Hobbs, R.E. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc. Diagn. 1990, 21, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Derrick, M.J.; Moreno-Cabral, R.J. Anomalous origin of the left anterior descending artery from the pulmonary artery. J. Card. Surg. 1991, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Wesselhoeft, H.; Fawcett, J.S.; Johnson, A.L. Anomalous origin of the left coronary artery from the pulmonary trunk. Its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation 1968, 38, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Azakie, A.; Russell, J.L.; McCrindle, B.W.; Van Arsdell, G.S.; Benson, L.N.; Coles, J.G.; et al. Anatomic repair of anomalous left coronary artery from the pulmonary artery by aortic reimplantation: Early survival, patterns of ventricular recovery and late outcome. Ann. Thorac. Surg. 2003, 75, 1535–1541. [Google Scholar] [CrossRef] [PubMed]

© 2013 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Robert, J.; Kadner, A.; Windecker, S.; Rosskopf, A.; Meier, B.; Schwerzmann, M. "Congenital" Chest Pain–Anomalous Origin of the Left Anterior Descending Coronary Artery from the Pulmonary Artery. Cardiovasc. Med. 2013, 16, 247. https://doi.org/10.4414/cvm.2013.00179
Robert J, Kadner A, Windecker S, Rosskopf A, Meier B, Schwerzmann M. "Congenital" Chest Pain–Anomalous Origin of the Left Anterior Descending Coronary Artery from the Pulmonary Artery. Cardiovascular Medicine. 2013; 16(9):247. https://doi.org/10.4414/cvm.2013.00179
Chicago/Turabian StyleRobert, Jens, Alexander Kadner, Stephan Windecker, Andrea Rosskopf, Bernhard Meier, and Markus Schwerzmann. 2013. ""Congenital" Chest Pain–Anomalous Origin of the Left Anterior Descending Coronary Artery from the Pulmonary Artery" Cardiovascular Medicine 16, no. 9: 247. https://doi.org/10.4414/cvm.2013.00179
APA StyleRobert, J., Kadner, A., Windecker, S., Rosskopf, A., Meier, B., & Schwerzmann, M. (2013). "Congenital" Chest Pain–Anomalous Origin of the Left Anterior Descending Coronary Artery from the Pulmonary Artery. Cardiovascular Medicine, 16(9), 247. https://doi.org/10.4414/cvm.2013.00179



