Case report
A 13-year-old Caucasian boy in good health was admitted to our hospital after a four-day history of febrile watery diarrhoea with abdominal pain, vomiting, dehydration and a rash. Because of asthenia and a high systemic inflammatory reaction (C-reactive protein [CRP] 254 mg/l), intravenous rehydration and treatment with ceftriaxone and metronidazole were started. After three days, the abdominal pain subsided, but the fever, headache and intense fatigue continued, with temperature spikes up to 39.5 °C. Blood culture as well as faecal culture were negative, as were abdominal ultrasound, rheumatoid factor, antinuclear antibodies and antineutrophil cytoplasmic antibodies (ANCAs).
On the seventh day, he developed bilateral conjunctival injection, a sore throat with a “strawberry” tongue, and redness and fissuring of the lips.
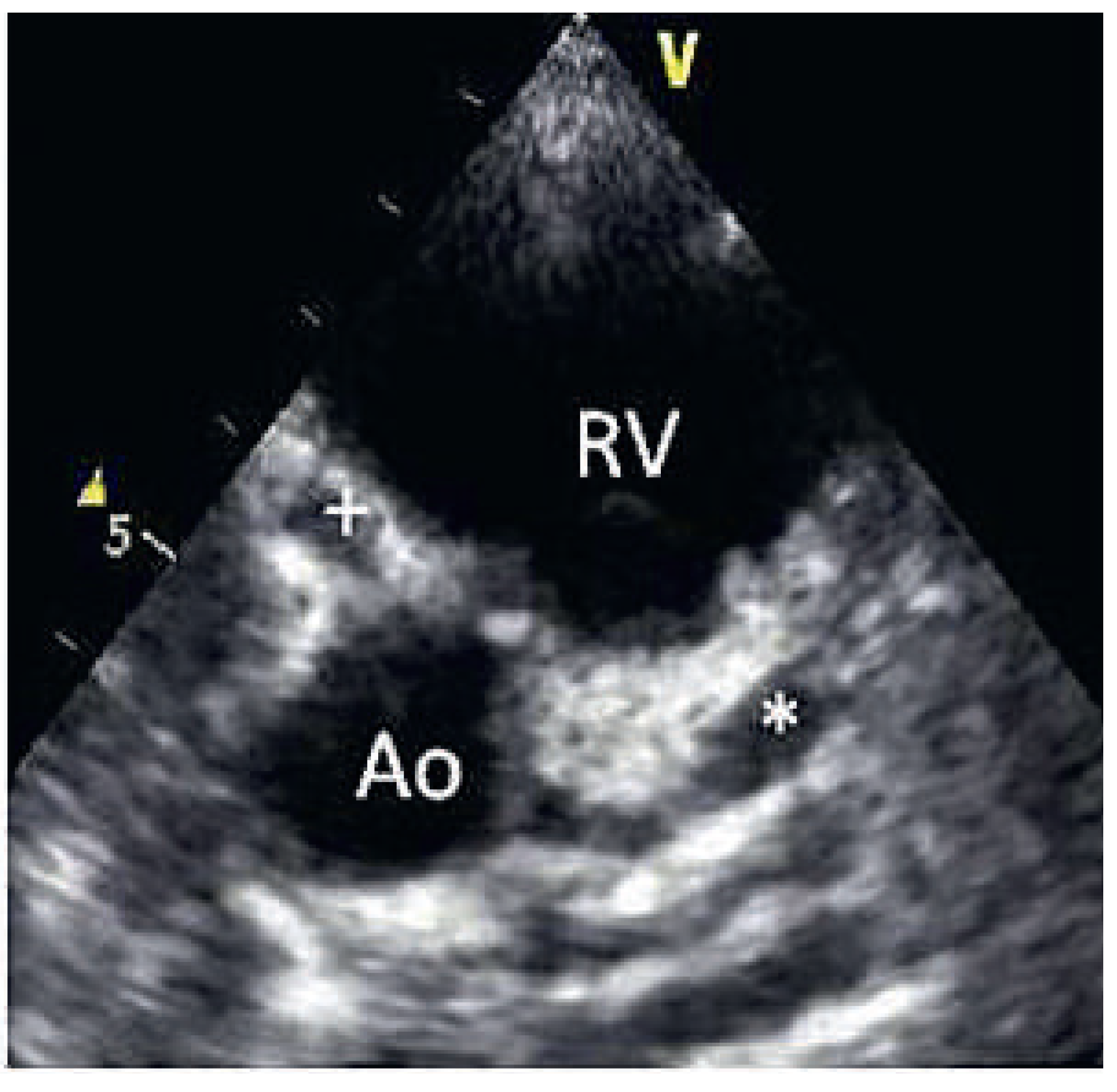
On the tenth day, we performed transthoracic echocardiography (
Figure 1 and
Figure 2), which revealed normal biventricular size and function, without hypokinesia. There was no pericardial effusion, no valvular disease, but a proximal enlargement of the left anterior descending coronary artery (LAD) was visualised. On the same day, because all these elements suggested Kawasaki disease (KD), treatment with intravenous polyvalent immunoglobulin (IVIG 2 g/kg as a single infusion: Privigen 70 g) and high-dose aspirin (80 to 100 mg/kg/d: 750 mg four times a day for eight days) was started, with rapid resolution of symptoms and apyrexia within 24 hours. Cardiac computed tomography (CT;
Figure 3) showed three-vessel aneurysmal coronary artery disease (CAD) including a giant aneurysm of the proximal LAD (9 mm), an aneurysm of the circumflex artery (6 mm) and two aneurysms of the right coronary artery (4 and 5 mm). There was no vascular involvement in the cerebral and abdominal magnetic resonance imaging (MRI).
The patient was discharged with combined therapy of aspirin 100 mg/d and oral anticoagulation (international normalised ratio [INR]: 2–3). Coronary angiography six months later, showed partial remodelling with persistence of the LAD aneurysm (
Figure 4). He underwent an exercise treadmill test nine months after disease onset. He achieved 92% of the maximum predicted heart rate without symptoms. The absence of ischaemia was confirmed with a positron emission tomography (PET) CT at the one-year follow-up.
The therapeutic plan and the follow-up diagnostic studies are the following: long-term dual therapy with aspirin and sintrom, biannual cardiac evaluation with echocardiogram and ECG, and annual exercise treadmill testing alternating with a myocardial scintigraphy test.
Discussion
Epidemiology [1]
KD or mucocutaneous lymph node syndrome is an acute self-limiting vasculitis of early childhood, which was first described in 1967 in Japan by Dr T. Kawasaki. The mean annual incidence is 216/100,000 children <5 years old in Japan, compared with an incidence of 21 and 9 per 100,000 children in the United States and England, respectively. KD occurs worldwide and has become the second most common systemic vasculitis in childhood after Henoch–Schönlein purpura. It has surpassed acute rheumatic fever as the leading cause of acquired heart disease in children in western countries. KD occurs more commonly during winter, is more common in boys, with a ratio of 1.5:1, and more commonly affects children younger than 5 years old, with a mean age of 1 year. KD rarely occurs in adolescents and young adults, with a hundred cases reported in the literature.
Aetiology and pathology [2,3]
Although an infectious origin for Kawasaki disease is suspected, no pathogen has been identified. The fact that the disease is more frequent in Japan and occurs more frequently in people of Asian ancestry living in other parts of the world suggests that both genetic susceptibility and environmental factors may play a role in KD. It is now believed that KD represents a final pathway of an immune vascular reaction in susceptible people, triggered by a common infection.
From a pathological point of view, despite the important mucocutaneous findings, KD is best described as a generalised vasculitis that involves muscular arteries, particularly the coronary arteries (CAs). Other muscular arteries such as coeliac, mesenteric, femoral, renal and axillary arteries may also be involved. KD has two phases. In the early stage, there is an infiltration of the CA with inflammatory cells leading to necrosis of the media. In severely affected vessels, the internal and external elastic laminae can split, leading to aneurysm formation. In the late stage, after several weeks to months, arterial remodelling occurs with scar formation in the vessel wall and intimal proliferation leading to stenosis or occlusion.
Diagnosis [2,3]
The diagnosis of KD relies on clinical findings. Laboratory tests are useful to rule out other causes of unexplained fever, but are not specific for a KD diagnosis. In 2004, the American Heart Association (AHA) established clinical criteria to help clinician. The diagnosis of KD requires the presence of fever lasting at least five days without any other explanation, with at least four of the following five criteria:
bilateral conjunctival injections,
oral mucous membrane changes,
cervical lymphandenopathy (at least one lymph node >1.5 cm in diameter, usually unilateral),
polymorphous truncal exanthem,
peripheral extremity changes, characterised in the acute phase by erythema of the palms or soles, followed during the convalescent phase by periungual desquamation.
Not all patients with KD meet the classic diagnostic criteria. The incomplete presentation of KD (fever lasting more than five days and two or three of the AHA clinical criteria), as in our case, is more common in infants aged <6 months or children aged >5 years and young adults, and is associated with more frequent and severe cardiac involvement. As in our case report, gastrointestinal involvement with nausea, diarrhoea and liver function abnormalities is frequently described in the literature at disease onset. Abdominal manifestations may be so acute and severe as to mimic appendicitis or pancreatitis, sometimes leading to surgical interventions.
Cardiac findings and initial evaluation [3]
Cardiovascular manifestations are the leading cause of long-term morbidity and mortality. The major complication of KD is coronary artery aneurysm (CAA), which usually develops in the first to sixth week. Myocarditis can occur during the acute phase. Pericardial effusion, mild mitral regurgitation and mild aortic root dilatation are less frequently described. In all patients with suspected KD, a cardiological evaluation with an ECG and an echocardiogram should be performed. ECG may show arrhythmia, prolonged PR interval, or nonspecific ST and T wave changes.
Echocardiography, because it is noninvasive and has high sensitivity (90%) and specificity (95%) for detection of abnormalities of proximal coronary arteries, remains the gold standard cardiac imaging modality in children [
3]. It should be performed during the acute phase and repeated after two and eight weeks. If patients exhibit no coronary involvement by eight weeks, follow-up echocardiography is recommended at one year. If at any point the echocardiogram is abnormal, the patient should be referred for further anatomic evaluation and assessment for reversible ischaemia. The types of stress tests reported for children with KD include treadmill stress testing, myocardial scintigraphy [
3] as well as stress echocardiography. Myocardial scintigraphy is a well documented method for assessment of ischaemia, but has some limitations (exposure to radiation, balanced ischaemia [
4]). Dobutamine stress echocardiography is a safe and accurate diagnostic method for detection of reversible ischaemia in KD with sensitivity of 90% and specificity of 100% [
5].
Long-term follow-up and prognosis [7,8]
KD was initially thought to be benign, but later reports described a mortality of 1%, which is believed to be due to cardiac arrhythmia or massive acute myocardial infarction. The peak mortality occurs 15–45 days after the onset of fever. The prognosis is based on the severity of coronary involvement. Patients who do not develop CAAs recover fully. In those with CAAs, the severity of the aneurysms determines the prognosis. Small aneurysms (<5 mm) fully regress in most of the cases and rarely progress to stenotic lesions. Large CAAs and giant aneurysms (>8 mm, as in our case) show a wide range of remodelling processes. Thrombotic occlusion may occur within two years after the onset of the disease, whereas stenoses due to intimal proliferation at the aneurysm entrance or exit may appear even after 10–15 years.
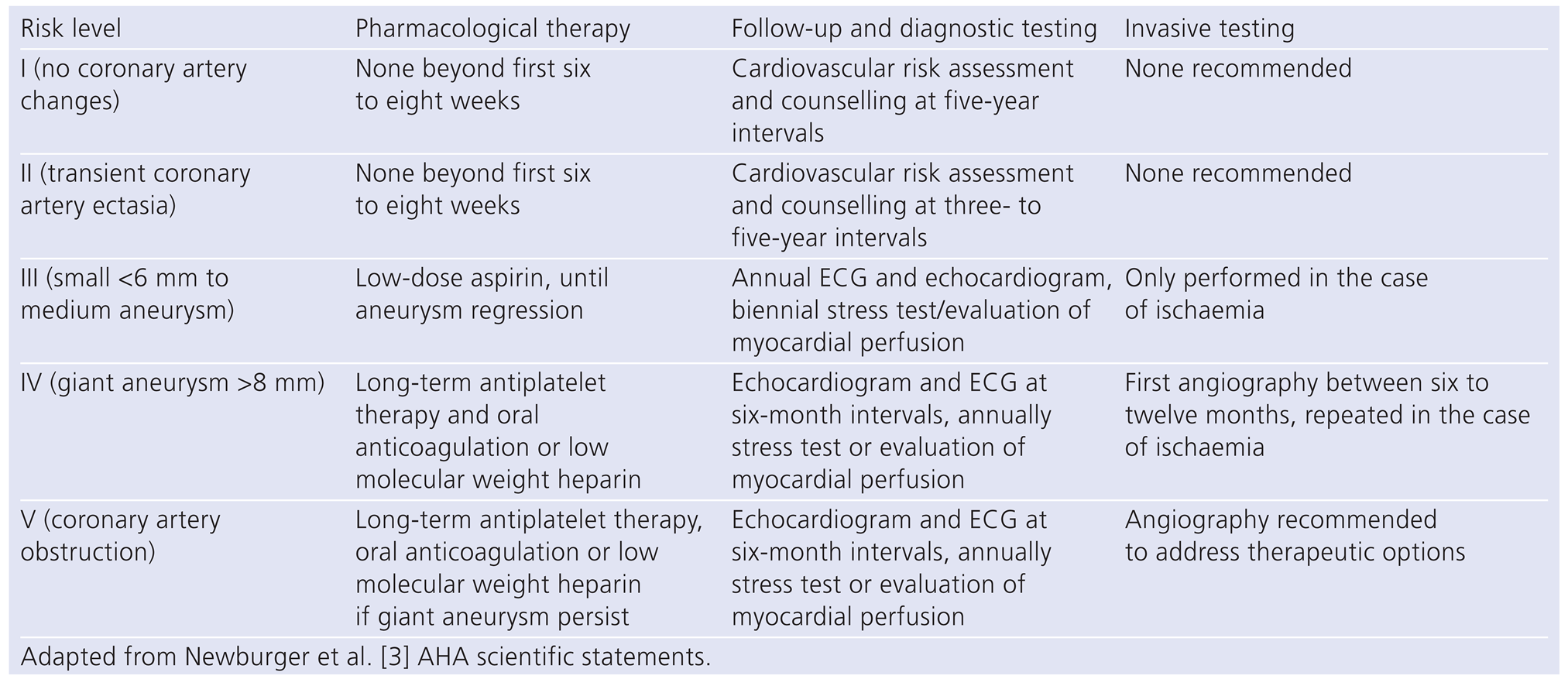
As patients with antecedent of KD will survive into adulthood, adult cardiologists are increasingly involved in their management. On one hand, they may deal with patients with known KD. In this case, they will have to determine which coronary abnormalities are associated with late cardiovascular events. Based on a consensus of experts, the AHA stratified the risk of events from level I to V and propose a management strategy adapted to each level (
Table 1).
On the other hand, adult cardiologists may have to deal with young adults presenting with an acute coronary syndrome, and whose cardiac imaging shows proximal coronary aneurysms with normal distal segments, or calcification of former remodelled aneurysms suggesting an undiagnosed KD [
9].
Treatment [2,3,7,8]
Intravenous immunoglobulin (IVIG 2 g/kg) is the mainstay of KD treatment. Its principal goal is to prevent coronary artery disease. IVIG, if initiated within the first ten days of the illness, has been shown to reduce the rate of CAA formation from greater than 20%–30% in untreated patients to less than 5% in treated patients. Aspirin has a synergistic effect with IVIG and is given during the acute phase in high doses (80–100 mg/kg/ day in four doses) as an anti-inflammatory agent and then in lower doses as an antiplatelet agent (3–5 mg/kg/ day) for six to eight weeks. The most common antithrombotic regimen for patients with giant aneurysms is low-dose aspirin combined with oral anticoagulation in order to prevent CAA thrombosis [
8]. Some patients continue to have fever 36 hours after the end of immunoglobulin administration and appear to be refractory to the treatment. This resistance can affect about 20%– 38% of the patients in some regions of the globe. These nonresponders should be first treated with a second IVIG course. In refractory forms, corticosteroid pulse therapy or treatment with infliximab (antitumour necrosis factor-alpha) could be an interesting alternative in the future [
2]. Coronary revascularisation is recommended for patients with giant or multiple CAAs or significant stenoses with ischaemia. Since most coronary artery lesions are located in proximal segments, distal graft target sites are easily accessible. Arterial grafts are preferred, because of their excellent potential for growth with the patient. Percutaneous transluminal coronary angioplasty (PTCA) or rotational ablation could be used as a bridge to surgery or to treat postoperative stenoses in the graft [
7].
Conclusion
KD can affect young adults with more frequent and severe cardiac involvement and with a worse prognosis. Its diagnosis relies on clinical criteria. KD usually resolves spontaneously, but may lead to serious complications. Coronary artery involvement is the major cause of morbidity and mortality. Aneurysms develop in 20%–30% of untreated patients and in 1%–5% of treated patients. Echocardiography is a good diagnostic tool for assessing cardiac involvement and looking for enlargement of the coronary arteries without any radiation exposure. Management includes early control of acute inflammation with IVIG and high-dose aspirin, monitoring for vascular aneurysms and coronary revascularisation in cases of demonstrated ischaemia.