Procalcitonin As A Prognostic Marker in Non-Infected Critically Ill Cardiovascular Patients
Summary
Introduction
Methods
Study design
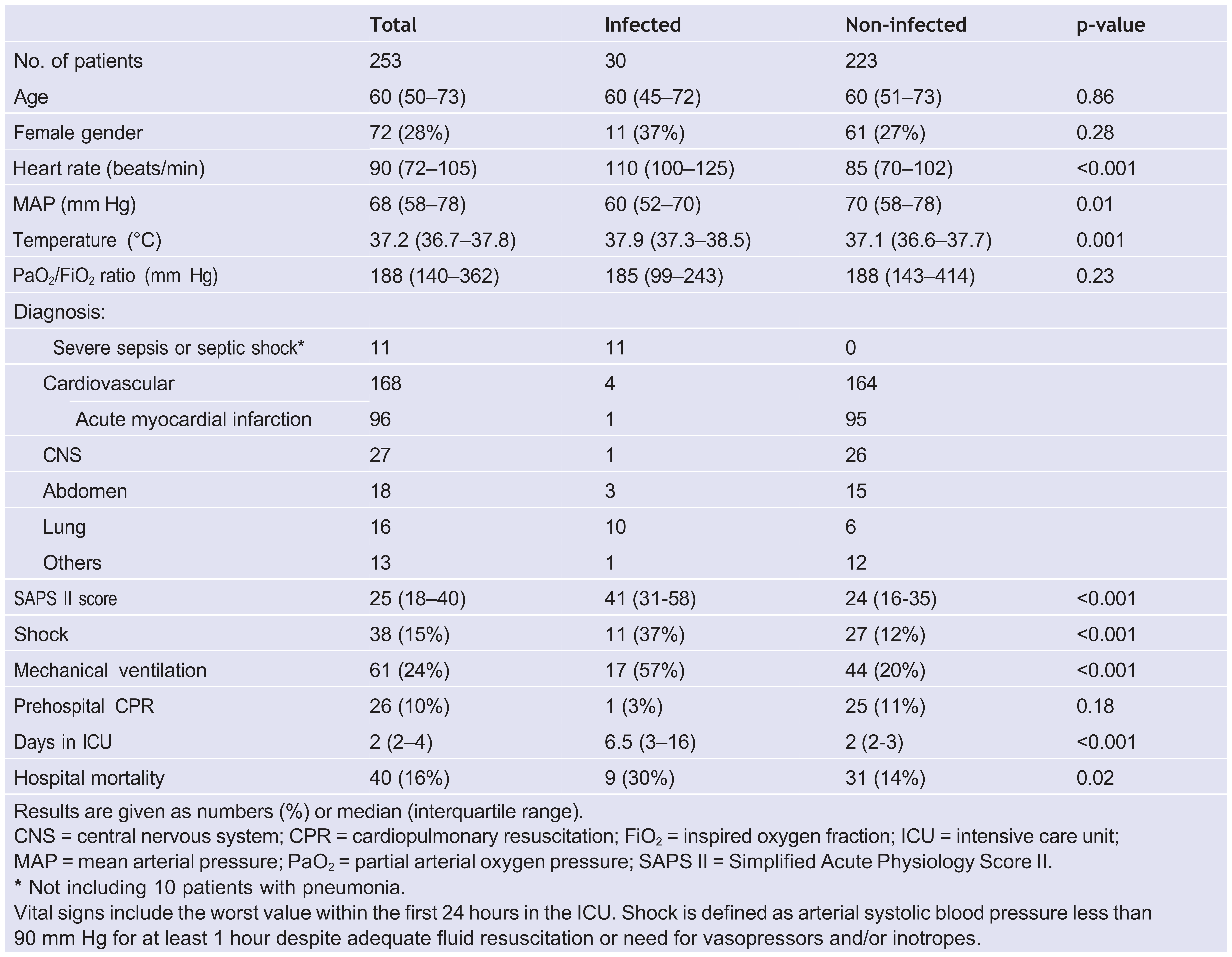
Patient characteristics
Monitoring and outcome parameters
Laboratory tests
Data analysis
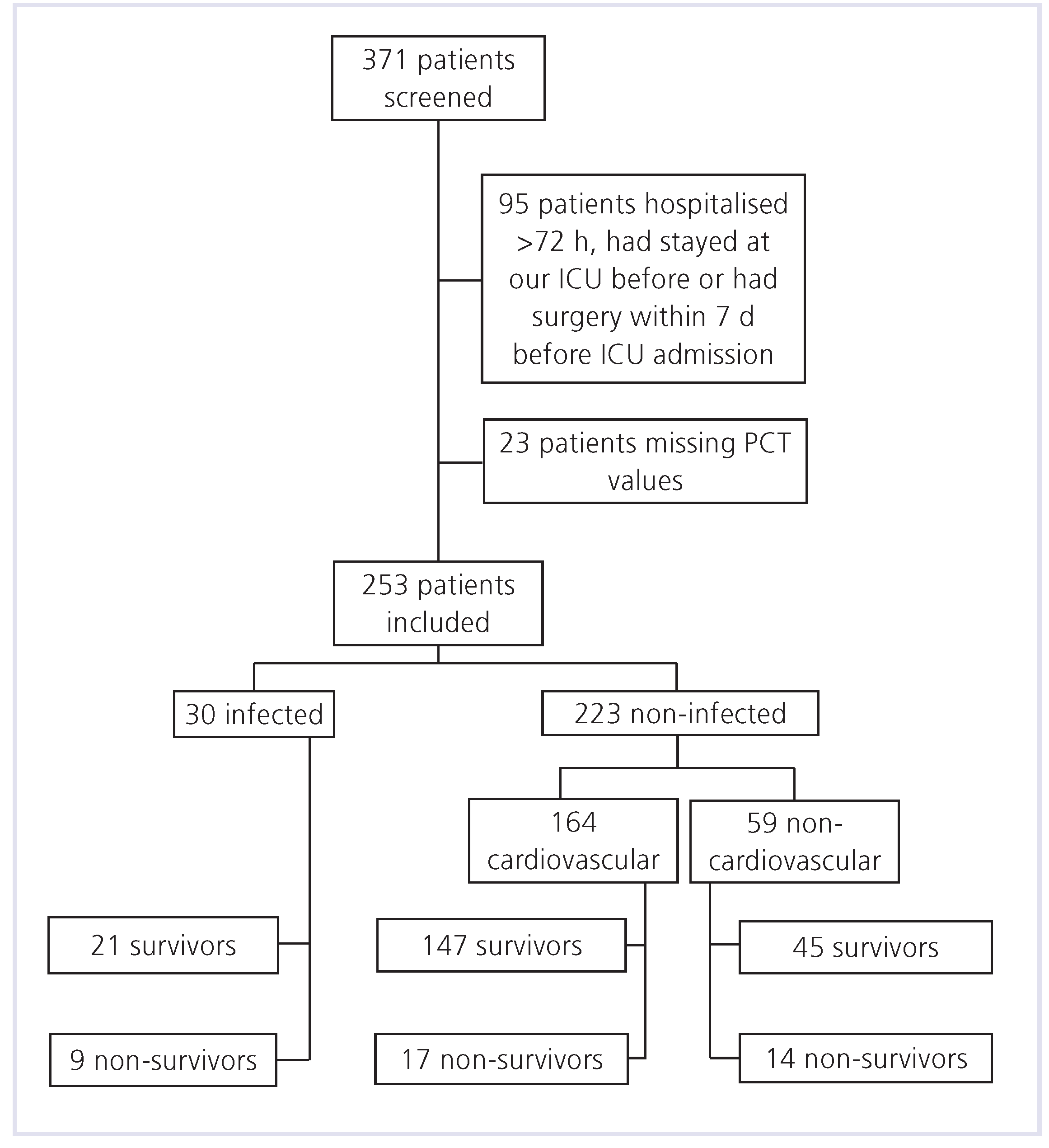
Results
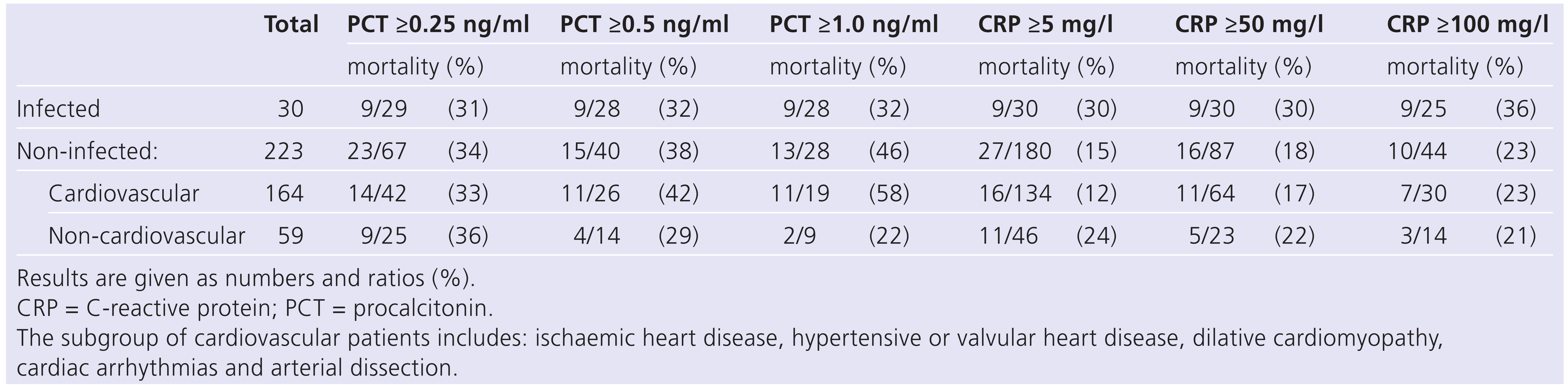
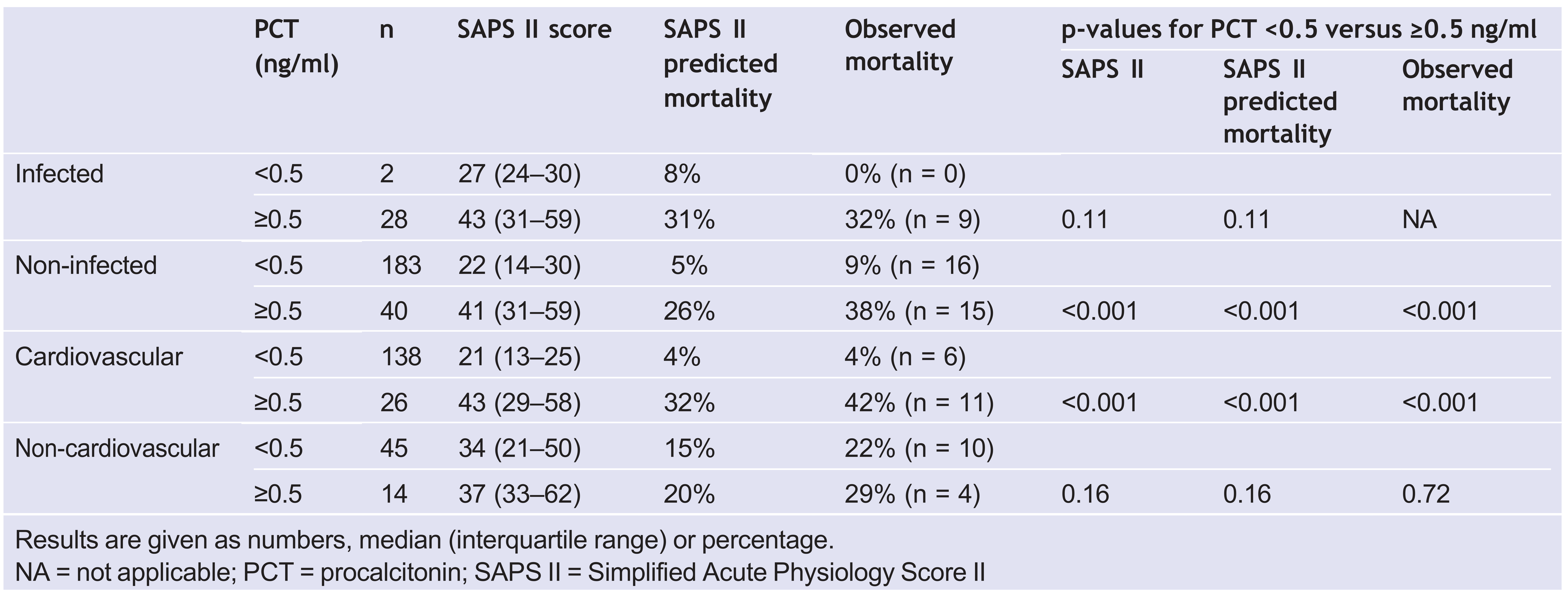
PCT measurements
CRP measurements
Outcome
Discussion
PCT plasma levels in non-infected patients
PCT as an outcome predictor
Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Schneider, C.P.; Yilmaz, Y.; Kleespies, A.; Jauch, K.W.; Hartl, W.H. Accuracy of procalcitonin for outcome prediction in unselected postoperative critically ill patients. Shock. 2009, 31, 568–573. [Google Scholar] [CrossRef]
- Brunkhorst, F.M.; Clark, A.L.; Forycki, Z.F.; Anker, S.D. Pyrexia, procalcitonin, immune activation and survival in cardiogenic shock: the potential importance of bacterial translocation. Int J Cardiol. 1999, 72, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Carsin, H.; Assicot, M.; Feger, F.; Roy, O.; Pennacino, I.; Le Bever, H.; et al. Evolution and significance of circulating procalcitonin levels compared with IL-6, TNF alpha and endotoxin levels early after thermal injury. Burns. 1997, 23, 218–224. [Google Scholar] [CrossRef]
- Schuetz, P.; Christ-Crain, M.; Muller, B. Biomarkers to improve diagnostic and prognostic accuracy in systemic infections. Curr Opin Crit Care. 2007, 13, 578–585. [Google Scholar] [CrossRef]
- Muller, B.; Becker, K.L.; Schachinger, H.; Rickenbacher, P.R.; Huber, P.R.; Zimmerli, W.; et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000, 28, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Oberhoffer, M.; Vogelsang, H.; Russwurm, S.; Hartung, T.; Reinhart, K. Outcome prediction by traditional and new markers of inflammation in patients with sepsis. Clin Chem Lab Med. 1999, 37, 363–368. [Google Scholar] [CrossRef]
- Bouadma, L.; Luyt, C.E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010, 375, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Dorge, H.; Schondube, F.A.; Dorge, P.; Seipelt, R.; Voss, M.; Messmer, B.J. Procalcitonin is a valuable prognostic marker in cardiac surgery but not specific for infection. Thorac Cardiovasc Surg. 2003, 51, 322–326. [Google Scholar]
- Kelly, D.; Khan, S.Q.; Dhillon, O.; Quinn, P.; Struck, J.; Squire, I.B.; et al. Procalcitonin as a prognostic marker in patients with acute myocardial infarction. Biomarkers. 2010, 15, 325–331. [Google Scholar] [CrossRef]
- Andrie, R.P.; Becher, U.M.; Frommold, R.; Tiyerili, V.; Schrickel, J.W.; Nickenig, G.; et al. Interleukin-6 is the strongest predictor of 30-day mortality in patients with cardiogenic shock due to myocardial infarction. Crit Care. 2012, 16, R152. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Eda, S.; Kaufmann, J.; Roos, W.; Pohl, S. Development of a new microparticleenhanced turbidimetric assay for C-reactive protein with superior features in analytical sensitivity and dynamic range. J Clin Lab Anal. 1998, 12, 137–144. [Google Scholar] [CrossRef]
- Steinbach, G.; Rau, B.; Debard, A.L.; Javourez, J.F.; Bienvenu, J.; Ponzio, A.; et al. Multicenter evaluation of a new immunoassay for procalcitonin measurement on the Kryptor System. Clin Chem Lab Med. 2004, 42, 440–449. [Google Scholar] [CrossRef]
- Kavsak, P.A.; MacRae, A.R.; Newman, A.M.; Lustig, V.; Palomaki, G.E.; Ko, D.T.; et al. Elevated C-reactive protein in acute coronary syndrome presentation is an independent predictor of long-term mortality and heart failure. Clin Biochem. 2007, 40, 326–329. [Google Scholar] [CrossRef]
- Bae, E.H.; Lim, S.Y.; Jeong, M.H.; Park, H.W.; Lim, J.H.; Hong, Y.J.; et al. Longterm predictive factors of major adverse cardiac events in patients with acute myocardial infarction complicated by cardiogenic shock. Korean J Intern Med. 2005, 20, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M.; Adina, H.; Schmidt, J. Correlation of procalcitonin and C-reactive protein to inflammation, complications, and outcome during the intensive care unit course of multiple-trauma patients. Crit Care. 2006, 10, R1. [Google Scholar] [CrossRef]
- Kafkas, N.; Venetsanou, K.; Patsilinakos, S.; Voudris, V.; Antonatos, D.; Kelesidis, K.; et al. Procalcitonin in acute myocardial infarction. Acute Card Care. 2008, 10, 30–36. [Google Scholar] [CrossRef]
- Senturk, T.; Cordan, J.; Baran, I.; Ozdemir, B.; Gullulu, S.; Aydinlar, A.; et al. Procalcitonin in patients with acute coronary syndrome: correlation with high-sensitive C-reactive protein, prognosis and severity of coronary artery disease. Acta Cardiol. 2007, 62, 135–141. [Google Scholar] [CrossRef]
- Linscheid, P.; Seboek, D.; Nylen, E.S.; Langer, I.; Schlatter, M.; Becker, K.L.; et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology. 2003, 144, 5578–5584. [Google Scholar] [CrossRef] [PubMed]
- Nijsten, M.W.; Olinga, P.; The, T.H.; de Vries, E.G.; Koops, H.S.; Groothuis, G.M.; et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med. 2000, 28, 458–461. [Google Scholar] [CrossRef]
- Linscheid, P.; Seboek, D.; Schaer, D.J.; Zulewski, H.; Keller, U.; Muller, B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004, 32, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Linscheid, P.; Seboek, D.; Zulewski, H.; Keller, U.; Muller, B. Autocrine/ paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005, 146, 2699–2708. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Muller, B. Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007, 30, 556–573. [Google Scholar] [CrossRef]
- Loebe, M.; Locziewski, S.; Brunkhorst, F.M.; Harke, C.; Hetzer, R. Procalcitonin in patients undergoing cardiopulmonary bypass in open heart surgery-first results of the Procalcitonin in Heart Surgery study (ProHearts). Intensive Care Med. 2000, 26 Suppl. 2, S193–S198. [Google Scholar] [CrossRef]
- Adib-Conquy, M.; Monchi, M.; Goulenok, C.; Laurent, I.; Thuong, M.; Cavaillon, J.M.; et al. Increased plasma levels of soluble triggering receptor expressed on myeloid cells 1 and procalcitonin after cardiac surgery and cardiac arrest without infection. Shock. 2007, 28, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Fries, M.; Kunz, D.; Gressner, A.M.; Rossaint, R.; Kuhlen, R. Procalcitonin serum levels after out-of-hospital cardiac arrest. Resuscitation. 2003, 59, 105–109. [Google Scholar] [CrossRef]
- Fries, M.; Stoppe, C.; Brucken, D.; Rossaint, R.; Kuhlen, R. Influence of mild therapeutic hypothermia on the inflammatory response after successful resuscitation from cardiac arrest. J Crit Care. 2009, 24, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Evans, T. Bacterial translocation in cardiopenic shock: the gastrointestinal tract as the motor of sepsis? Int J Cardiol. 1999, 72, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Gibot, S.; Cravoisy, A.; Kolopp-Sarda, M.N.; Bene, M.C.; Faure, G.; Bollaert, P.E.; et al. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med. 2005, 33, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Picariello, C.; Lazzeri, C.; Attana, P.; Chiostri, M.; Gensini, G.F.; Valente, S. The impact of admission procalcitonin on prognosis in acute coronary syndromes: a pilot study. Biomarkers. 2012, 17, 56–61. [Google Scholar] [CrossRef]
- Claeys, R.; Vinken, S.; Spapen, H.; ver Elst, K.; Decochez, K.; Huyghens, L.; et al. Plasma procalcitonin and C-reactive protein in acute septic shock: clinical and biological correlates. Crit Care Med. 2002, 30, 757–762. [Google Scholar] [CrossRef]
- Wunder, C.; Eichelbronner, O.; Roewer, N. Are IL-6, IL-10 and PCT plasma concentrations reliable for outcome prediction in severe sepsis? A comparison with APACHE III and SAPS II. Inflamm Res. 2004, 53, 158–163. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, C.H.; Yun, J.W.; Lee, Y.S. Predictive comparisons of procalcitonin (PCT) level, arterial ketone body ratio (AKBR), APACHE III score and multiple organ dysfunction score (MODS) in systemic inflammatory response syndrome (SIRS). Yonsei Med J. 2004, 45, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Novotny, A.; Emmanuel, K.; Matevossian, E.; Kriner, M.; Ulm, K.; Bartels, H.; et al. Use of procalcitonin for early prediction of lethal outcome of postoperative sepsis. Am J Surg. 2007, 194, 35–39. [Google Scholar] [CrossRef] [PubMed]


 |
 |
 |
© 2013 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Koning, J.; Ritter, S.; Maggiorini, M. Procalcitonin As A Prognostic Marker in Non-Infected Critically Ill Cardiovascular Patients. Cardiovasc. Med. 2013, 16, 321. https://doi.org/10.4414/cvm.2013.00197
Koning J, Ritter S, Maggiorini M. Procalcitonin As A Prognostic Marker in Non-Infected Critically Ill Cardiovascular Patients. Cardiovascular Medicine. 2013; 16(12):321. https://doi.org/10.4414/cvm.2013.00197
Chicago/Turabian StyleKoning, Judith, Simon Ritter, and Marco Maggiorini. 2013. "Procalcitonin As A Prognostic Marker in Non-Infected Critically Ill Cardiovascular Patients" Cardiovascular Medicine 16, no. 12: 321. https://doi.org/10.4414/cvm.2013.00197
APA StyleKoning, J., Ritter, S., & Maggiorini, M. (2013). Procalcitonin As A Prognostic Marker in Non-Infected Critically Ill Cardiovascular Patients. Cardiovascular Medicine, 16(12), 321. https://doi.org/10.4414/cvm.2013.00197




