Left Ventricular Apical Ballooning Syndrome with Extensive Myocardial Late Gadolinium Enhancement: Tako-Tsubo Cardiomyopathy, Perhaps Not as Benign as Previously Thought?
Summary
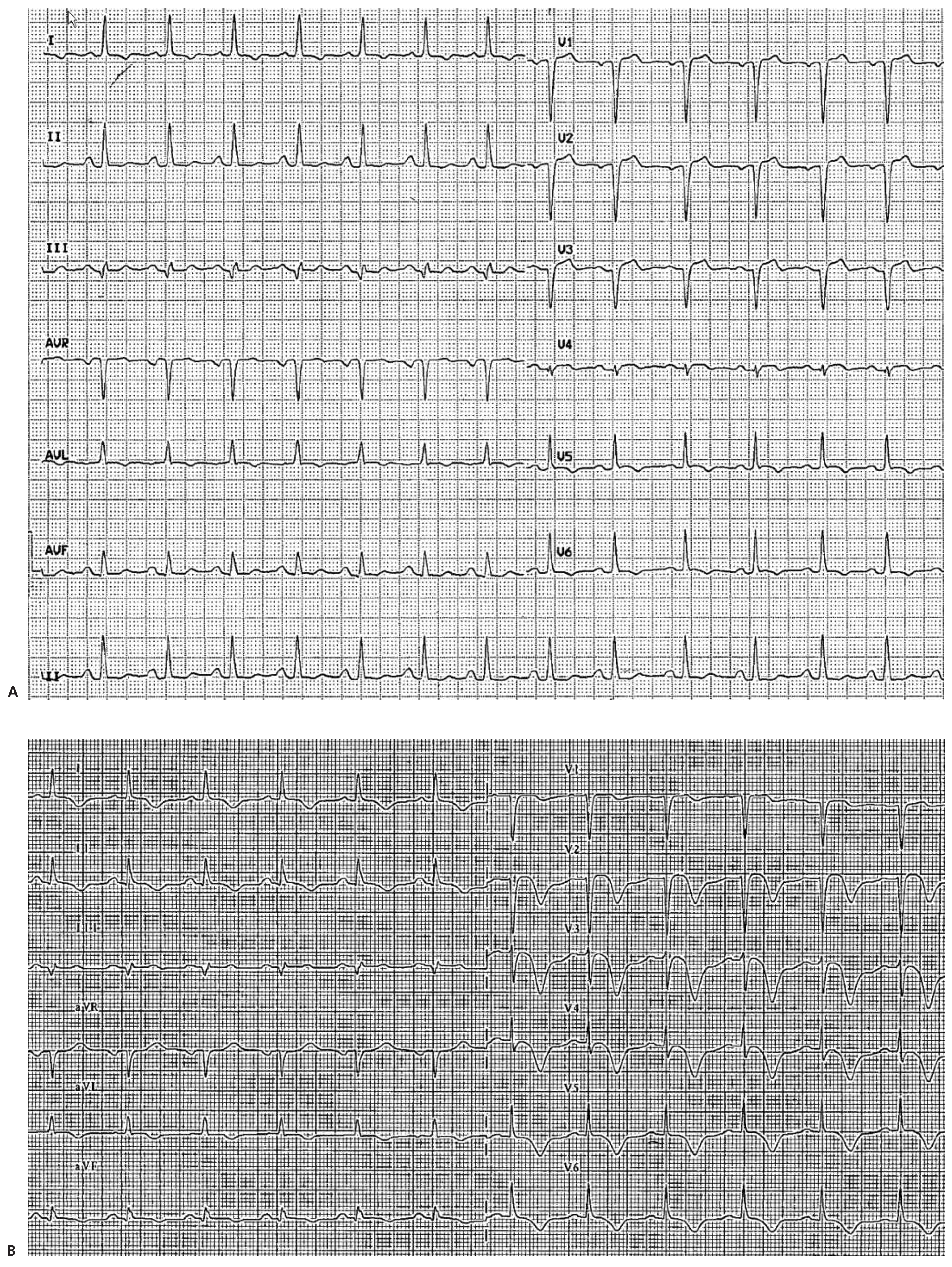
Case Report
Funding/Potential Competing Interests
References
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Behrendt, F.; Schindler, K.; Kivelitz, D.; Gutberlet, M.; Schuler, G.; et al. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J. 2008, 29, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Rolf, A.; Nef, H.M.; Möllmann, H.; Troidl, C.; Voss, S.; Conradi, G.; et al. Immunohistological basis of the late gadolinium enhancement phenomenon in tako-tsubo cardiomyopathy. Eur Heart J. 2009, 30, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Flett, A.; Westwood, M.; Davies, L.; Mathur, A.; Moon, J. The prognostic implications of Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging 2009, 2, 243–250. [Google Scholar] [CrossRef] [PubMed]



© 2012 by the author. Attribution-Non-Commercial-NoDerivatives 4.0.
Share and Cite
Gabus, V.; Schwitter, J.; Eeckhout, E.; Locca, D. Left Ventricular Apical Ballooning Syndrome with Extensive Myocardial Late Gadolinium Enhancement: Tako-Tsubo Cardiomyopathy, Perhaps Not as Benign as Previously Thought? Cardiovasc. Med. 2012, 15, 325. https://doi.org/10.4414/cvm.2012.00120
Gabus V, Schwitter J, Eeckhout E, Locca D. Left Ventricular Apical Ballooning Syndrome with Extensive Myocardial Late Gadolinium Enhancement: Tako-Tsubo Cardiomyopathy, Perhaps Not as Benign as Previously Thought? Cardiovascular Medicine. 2012; 15(11):325. https://doi.org/10.4414/cvm.2012.00120
Chicago/Turabian StyleGabus, Vincent, Juerg Schwitter, Eric Eeckhout, and Didier Locca. 2012. "Left Ventricular Apical Ballooning Syndrome with Extensive Myocardial Late Gadolinium Enhancement: Tako-Tsubo Cardiomyopathy, Perhaps Not as Benign as Previously Thought?" Cardiovascular Medicine 15, no. 11: 325. https://doi.org/10.4414/cvm.2012.00120
APA StyleGabus, V., Schwitter, J., Eeckhout, E., & Locca, D. (2012). Left Ventricular Apical Ballooning Syndrome with Extensive Myocardial Late Gadolinium Enhancement: Tako-Tsubo Cardiomyopathy, Perhaps Not as Benign as Previously Thought? Cardiovascular Medicine, 15(11), 325. https://doi.org/10.4414/cvm.2012.00120



