Iatrogenic Left Main Stem Stenosis After Surgical Aortic Valve Replacement
Abstract
Case presentations
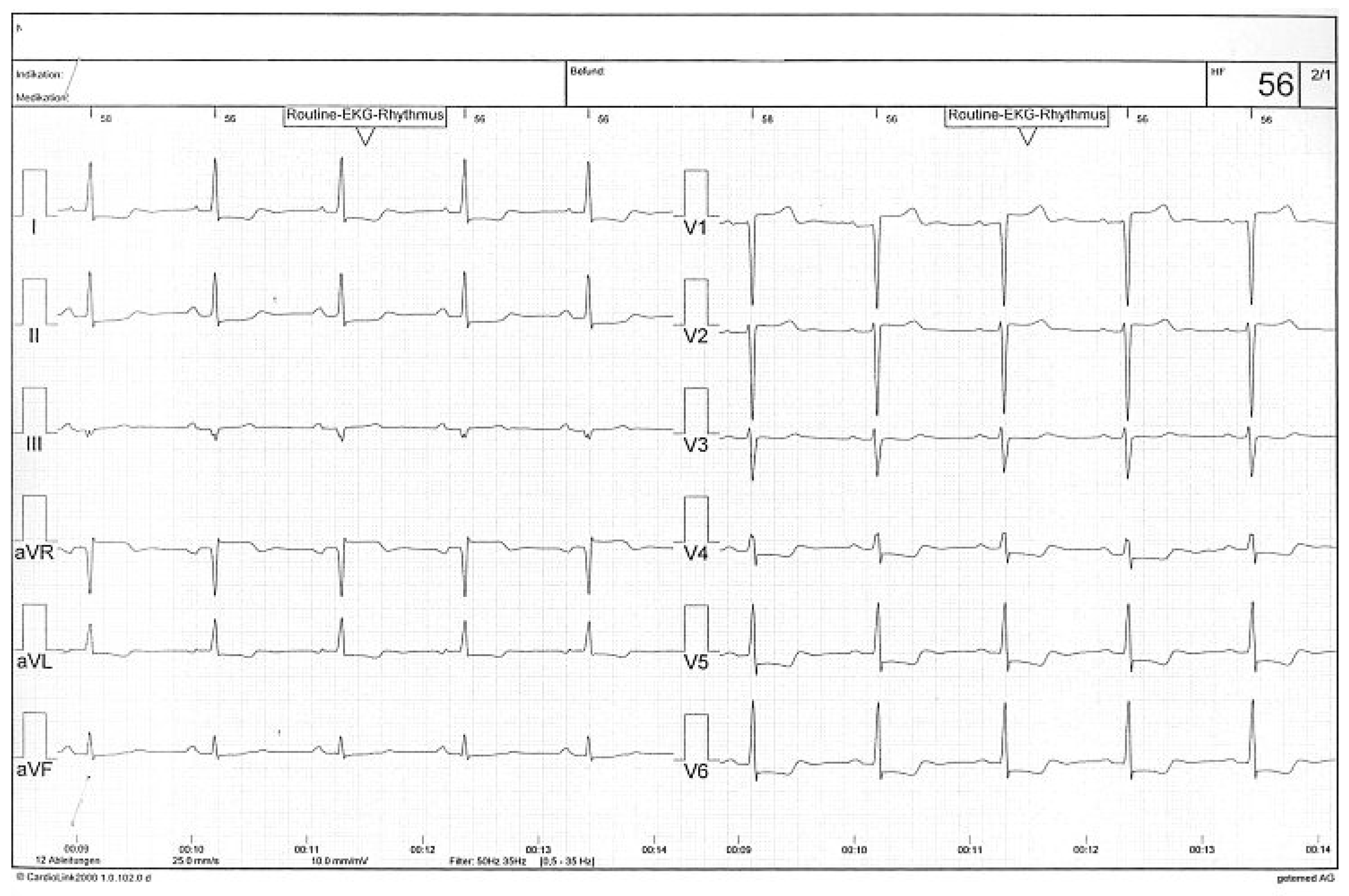
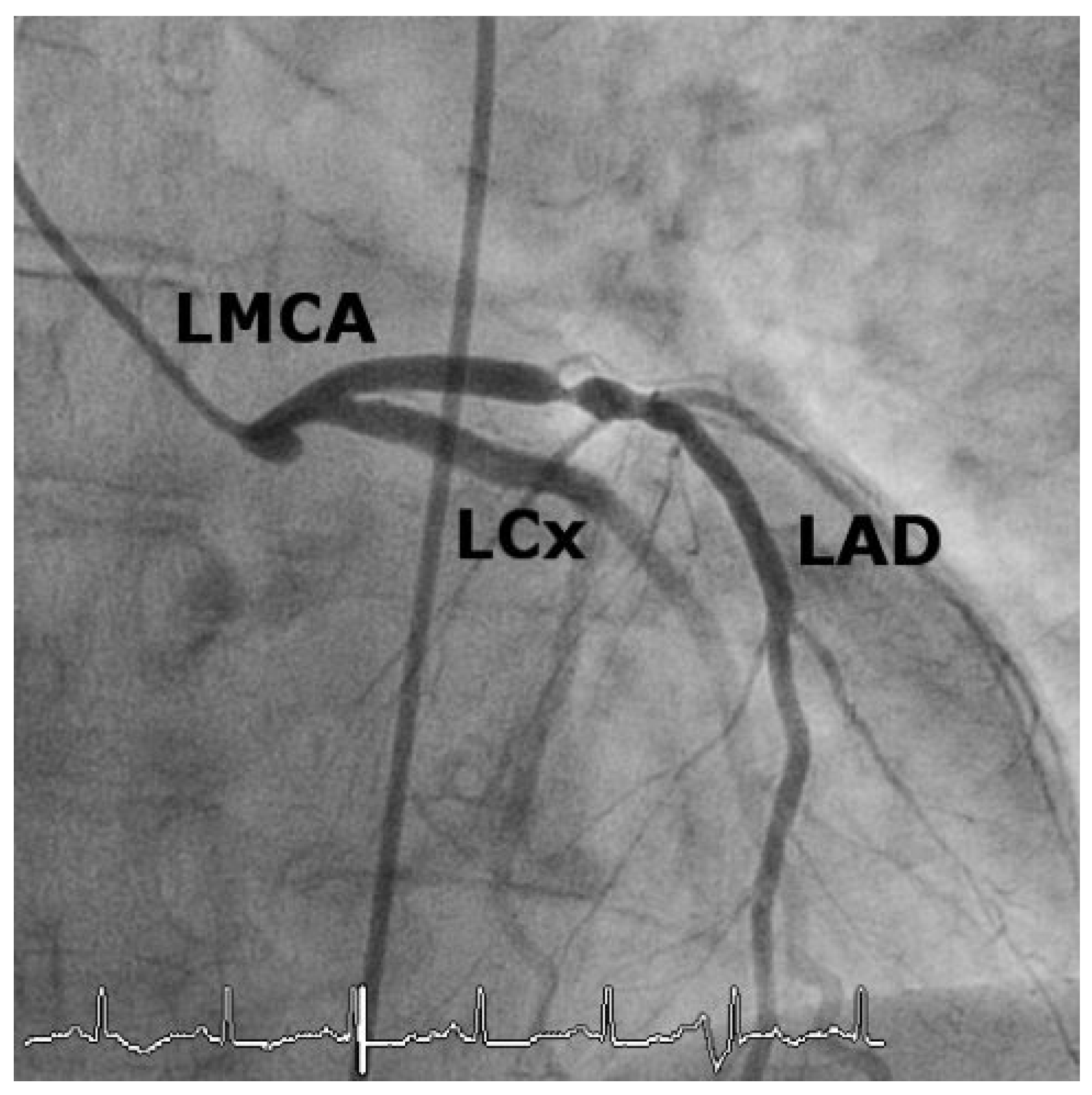
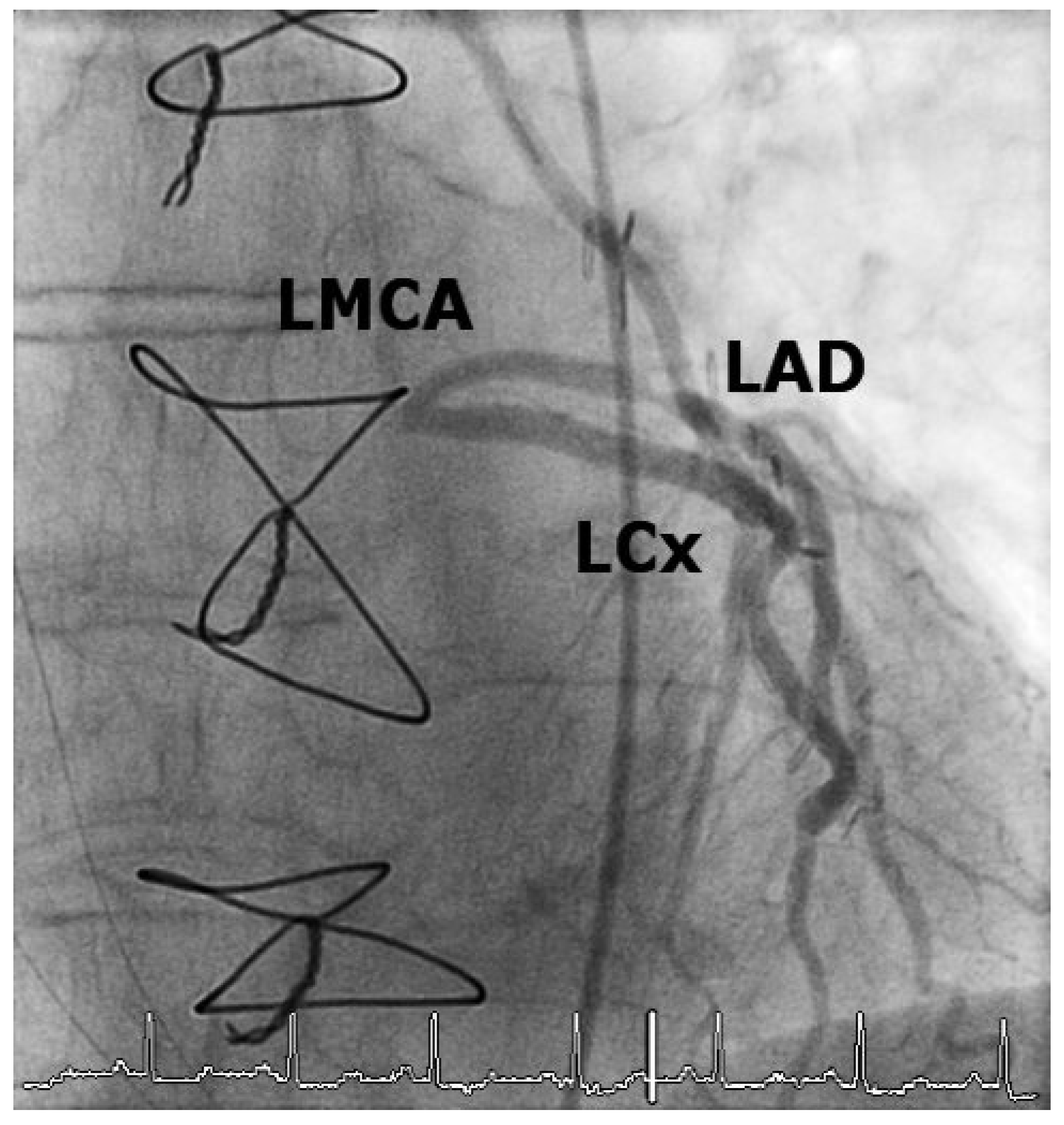
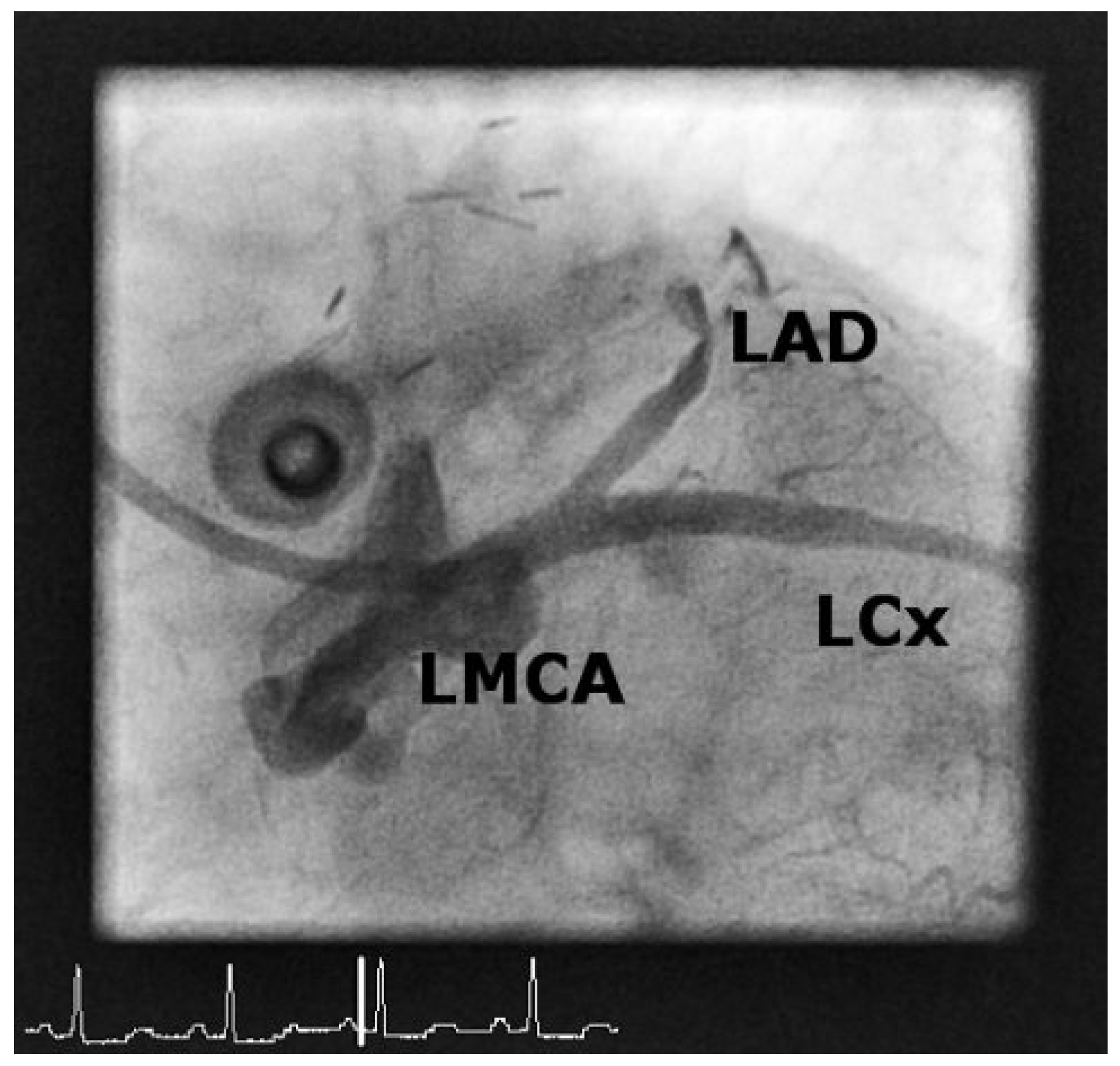
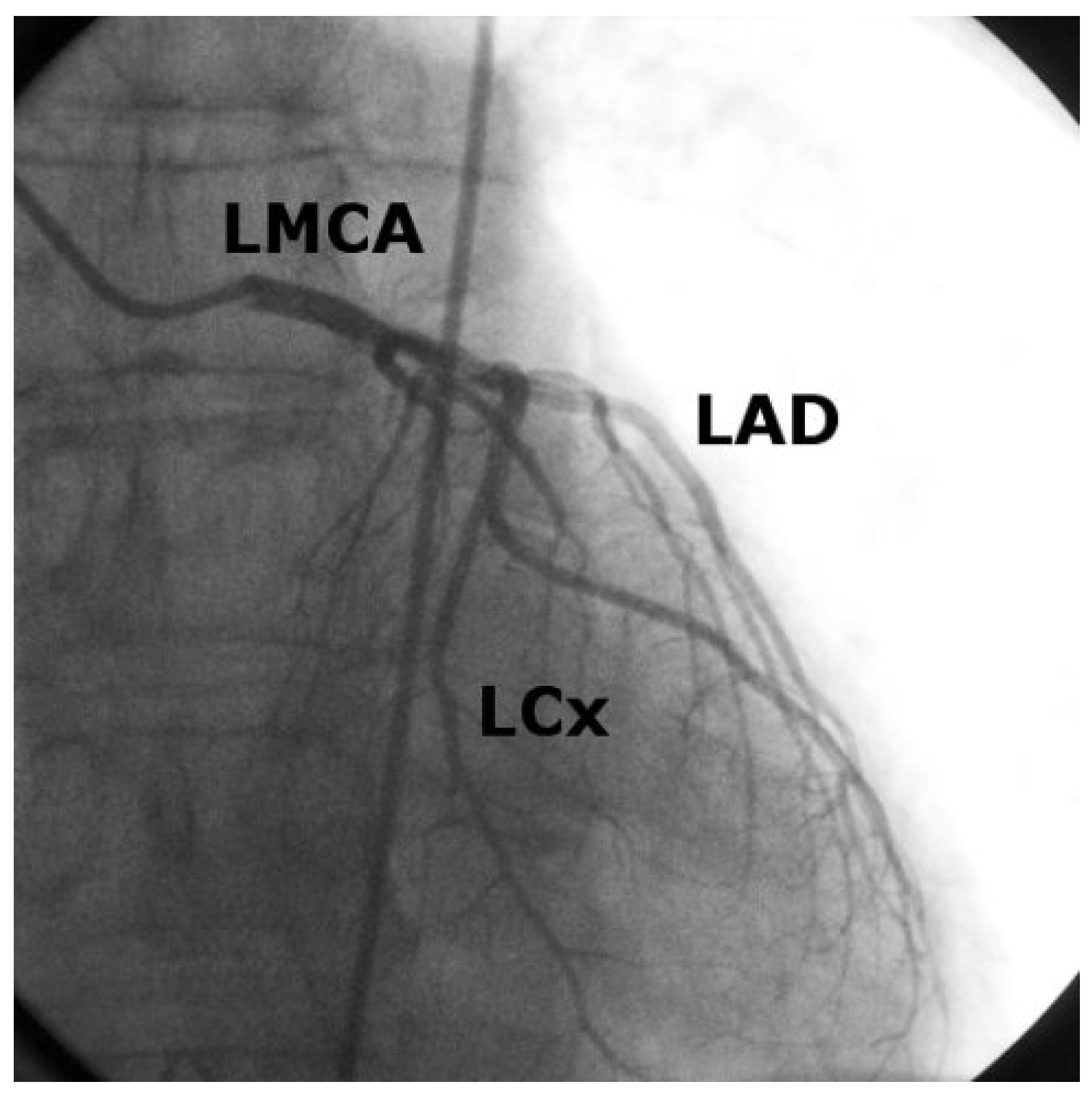
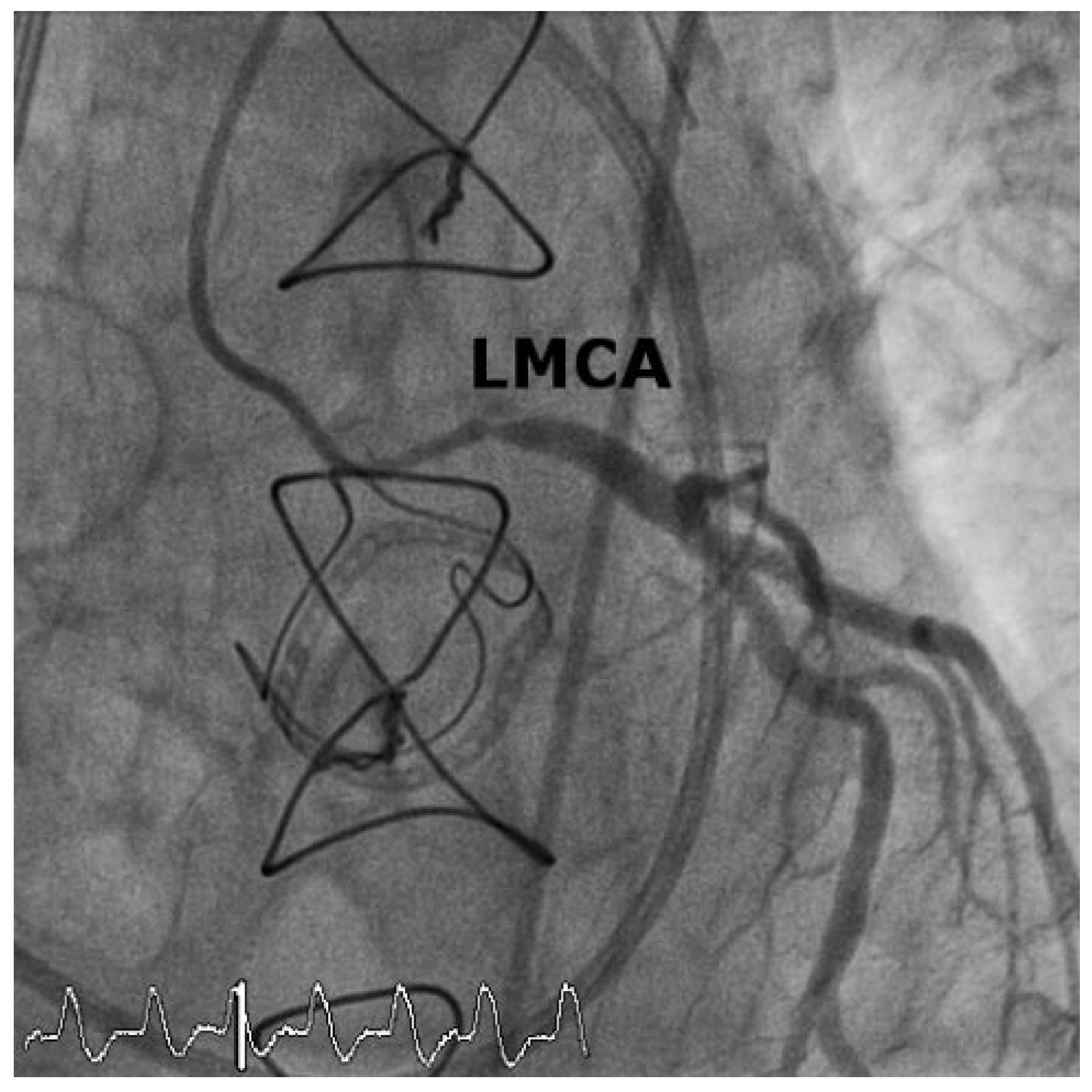
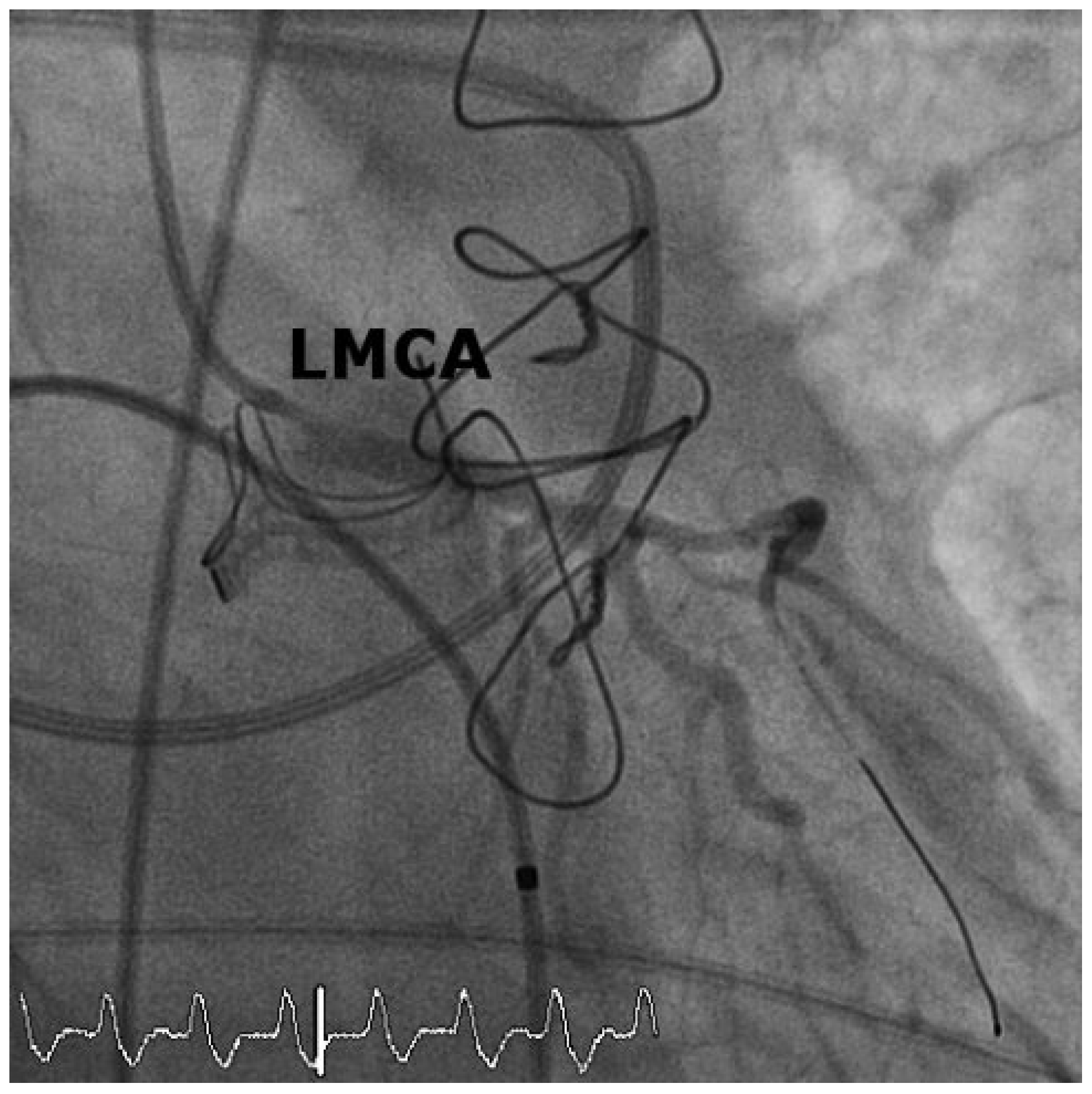
Case 1
Case2
Discussion
Conclusion
Conflicts of Interest
References
- Trimble, A.S.; Bigelow, W.G.; Wigle, E.D.; Silver, M.D. Coronary ostial stenosis: a late complication of coronary perfusion in open-heart surgery. J Thorac Cardiovasc Surg. 1969, 57, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.D.; Wigle, E.D.; Trimble, A.S.; Bigelow, W.G. Iatrogenic coronary ostial stenosis. Arch Pathol. 1969, 88, 73–77. [Google Scholar] [PubMed]
- Force, T.L.; Raabe, D.S.; Jr Coffin, L.H.; DeMeules, J.D. Coronary ostial stenosis following aortic valve replacement without continuous coronary perfusion. J Thorac Cardiovasc Surg. 1980, 80, 637–641. [Google Scholar] [PubMed]
- Simarro Martin, E.; Moris de la Tassa, C.; Rodriguez Lambert, J.L.; Sieres Felgueres, M.; Gutierrez Rodriguez, J.A.; Cortina Llosa, A. Percutaneous transluminal coronary angioplasty in a case of iatrogenic stenosis of the left coronary ostium. Rev Esp Cardiol. 1984, 37, 366–367. [Google Scholar] [PubMed]
- Winkelmann, B.R.; Ihnken, K.; Beyersdorf, F.; Eckel, L.; Skupin, M.; Marz, W.; et al. Left main coronary artery stenosis after aortic valve replacement: genetic disposition for accelerated arteriosclerosis after injury of the intact human coronary artery? Coron Artery Dis. 1993, 4, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Chavanon, O.; Carrier, M.; Cartier, R.; Hebert, Y.; Pellerin, M.; Perrault, L.P. Early reoperation for iatrogenic left main stenosis after aortic valve replacement: a perilous situation. Cardiovasc Surg. 2002, 10, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Tsukiji, M.; Akasaka, T.; Wada, N.; Okahashi, N.; Kume, T.; Yoshitani, H.; et al. Bilateral coronary ostial stenosis after aortic valve replacement with freestyle stentless bioprosthesis: a case report. J Cardiol. 2004, 44, 207–213. [Google Scholar] [PubMed]
- Hadjimiltiades, S.; Harokopos, N.; Papadopoulos, C.; Gourassas, I.; Spanos, P.; Louridas, G. Left main coronary artery stenosis after aortic valve replacement. Hellenic J Cardiol. 2005, 46, 306–309. [Google Scholar] [PubMed]
- Chieffo, A.; Park, S.J.; Valgimigli, M.; Kim, Y.H.; Daemen, J.; Sheiban, I.; et al. Favorable long-term outcome after drug-eluting stent implantation in nonbifurcation lesions that involve unprotected left main coronary artery: a multicenter registry. Circulation. 2007, 116, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Dangas, G.D.; Solinas, E.; Aoki, J.; Parise, H.; Kimura, M.; et al. Effectiveness of drug-eluting stent implantation for patients with unprotected left main coronary artery stenosis. Am J Cardiol. 2008, 101, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Morice, M.C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009, 360, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Morrow, A.G. Late postoperative pathological findings after cardiac valve replacement. Circulation. 1967, 35 (Suppl. 4), I48–62. [Google Scholar] [CrossRef] [PubMed]
- Fishman, N.H.; Youker, J.E.; Roe, B.B. Mechanical injury to the coronary arteries during operative cannulation. Am Heart J. 1968, 75, 26–33. [Google Scholar] [CrossRef] [PubMed]

© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Robert, J.; Tüller, D.; Windecker, S. Iatrogenic Left Main Stem Stenosis After Surgical Aortic Valve Replacement. Cardiovasc. Med. 2011, 14, 101. https://doi.org/10.4414/cvm.2011.01578
Robert J, Tüller D, Windecker S. Iatrogenic Left Main Stem Stenosis After Surgical Aortic Valve Replacement. Cardiovascular Medicine. 2011; 14(3):101. https://doi.org/10.4414/cvm.2011.01578
Chicago/Turabian StyleRobert, Jens, David Tüller, and Stephan Windecker. 2011. "Iatrogenic Left Main Stem Stenosis After Surgical Aortic Valve Replacement" Cardiovascular Medicine 14, no. 3: 101. https://doi.org/10.4414/cvm.2011.01578
APA StyleRobert, J., Tüller, D., & Windecker, S. (2011). Iatrogenic Left Main Stem Stenosis After Surgical Aortic Valve Replacement. Cardiovascular Medicine, 14(3), 101. https://doi.org/10.4414/cvm.2011.01578



