Posterior Probabilities in Sequential Testing Improve Clinical Cardiovascular Risk Prediction Using Carotid Total Plaque Area and C-Statistics
Abstract
Background
Methods
Patients
Clinical and laboratory measurements
Risk assessment using NCEP III risk algorithm and outcome data
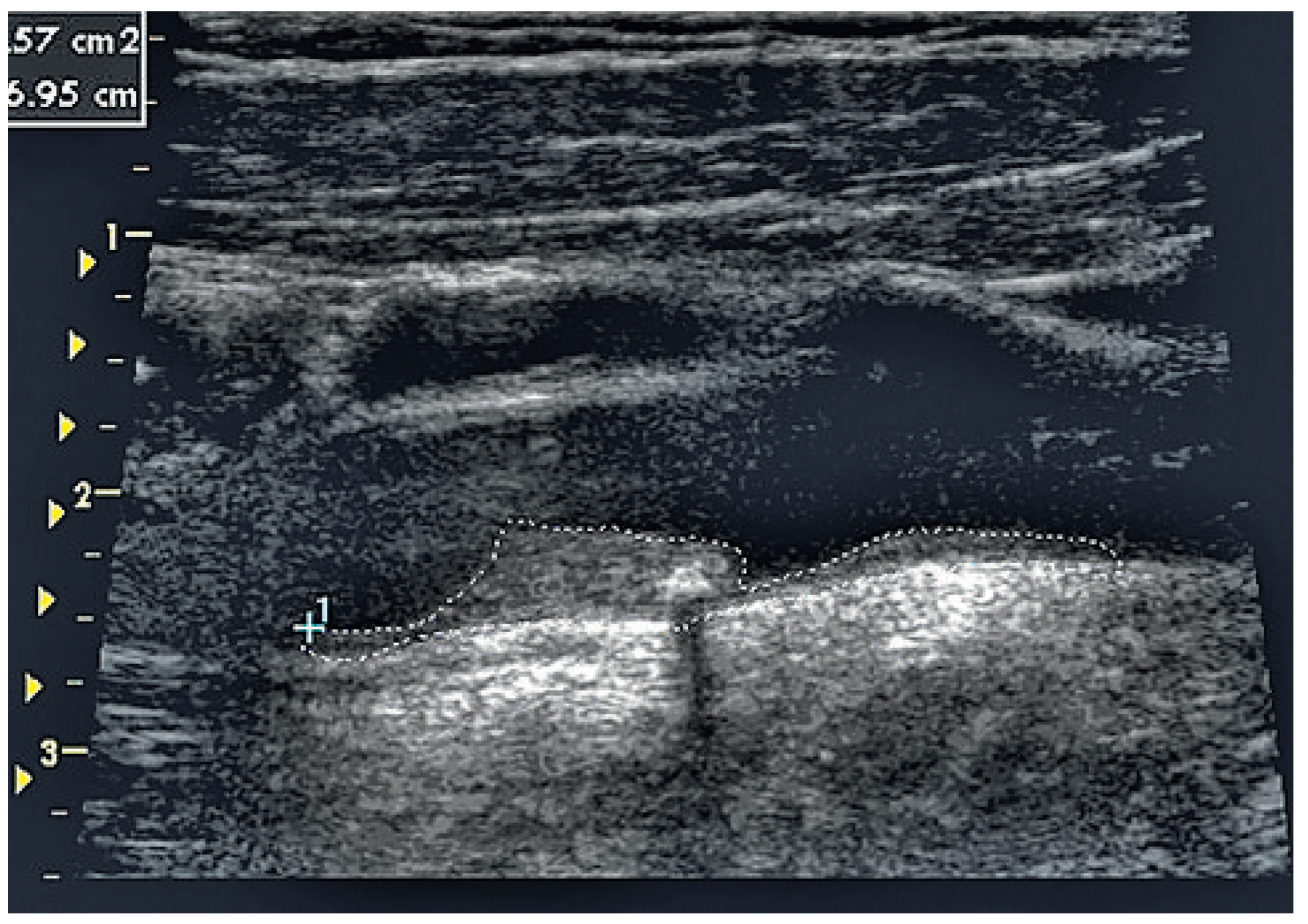
Imaging method and risk assessment using total plaque area of carotid arteries.
Statistical methods
- Incaseofanegativetest(TPA <0.18 cm2):
- PTPneg:[PV x(1−SE)]/[PV x(1–SE)+SP x(1–PV)]
- Incaseofapositivetest(TPA≥0.18cm2):
- PTPpos:(PVxSE)/[PVxSE+(1–PV)x(1–SP)]
Results
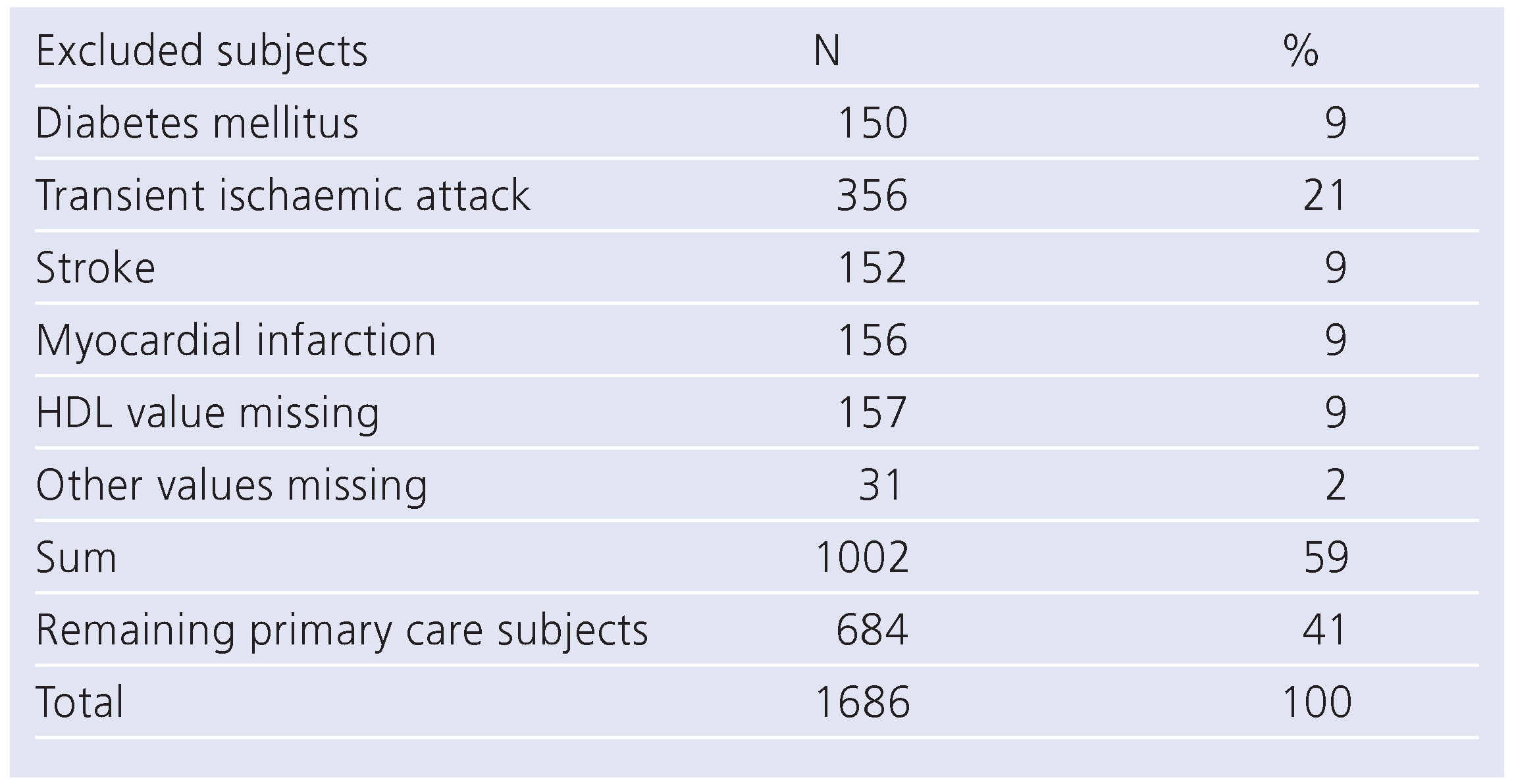
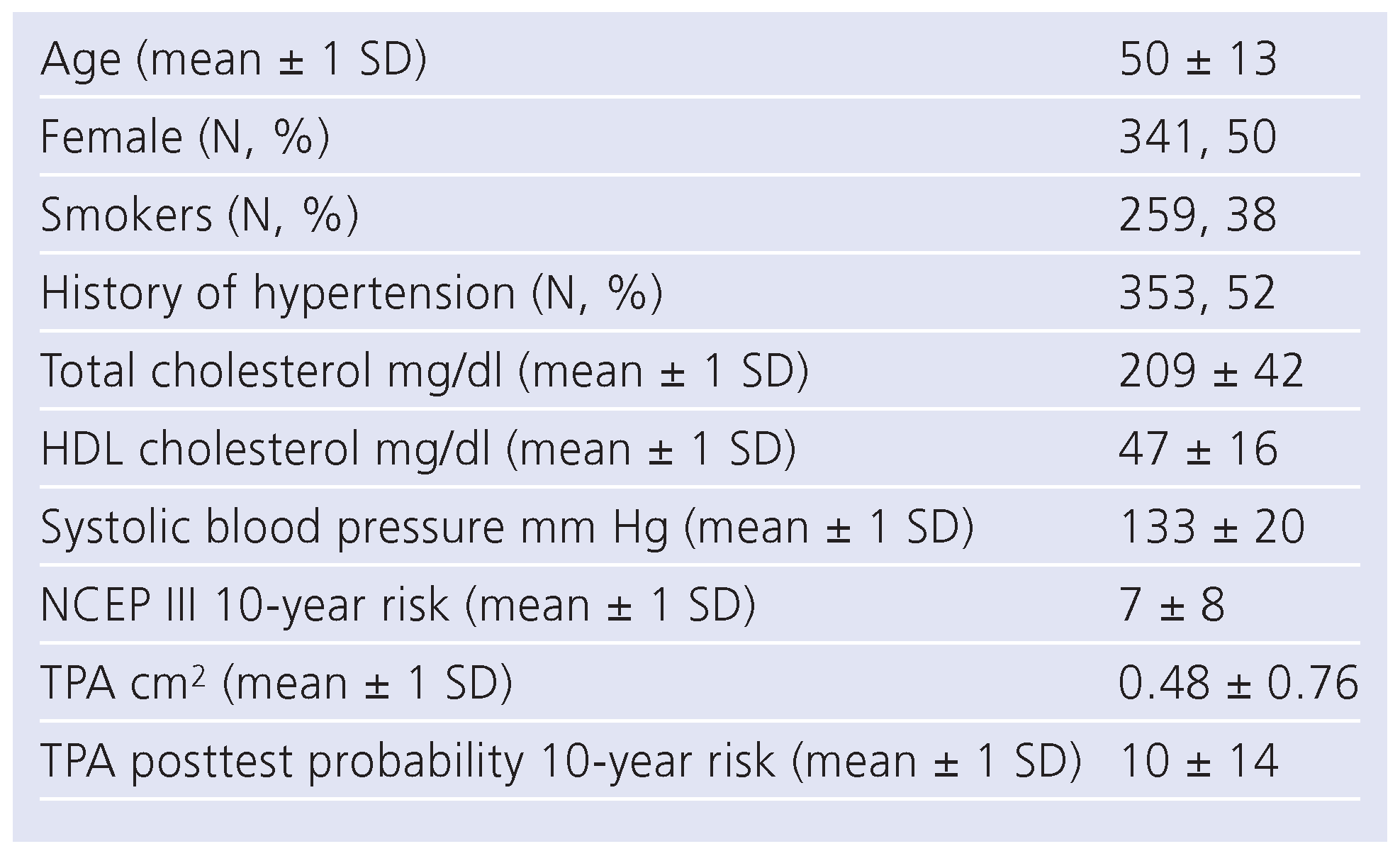
Patients
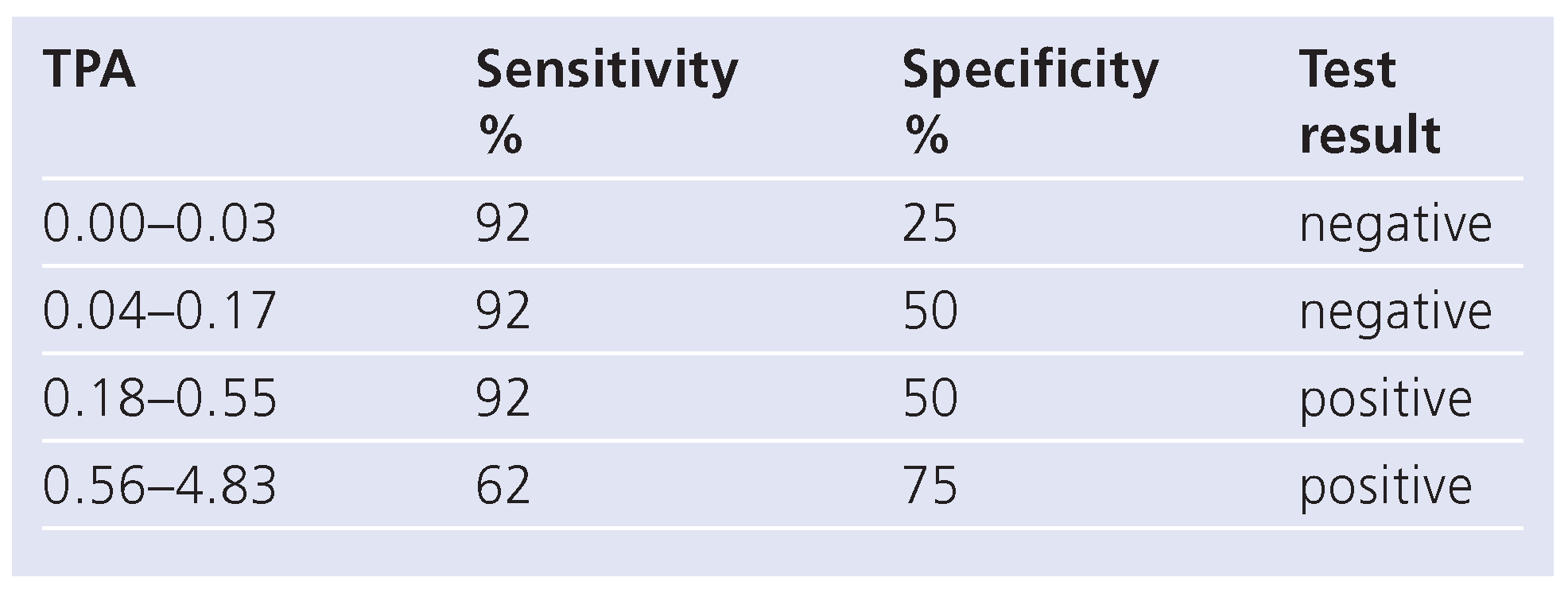
Sensitivities and specificities of NCEP III, TPA and TPA-PTP
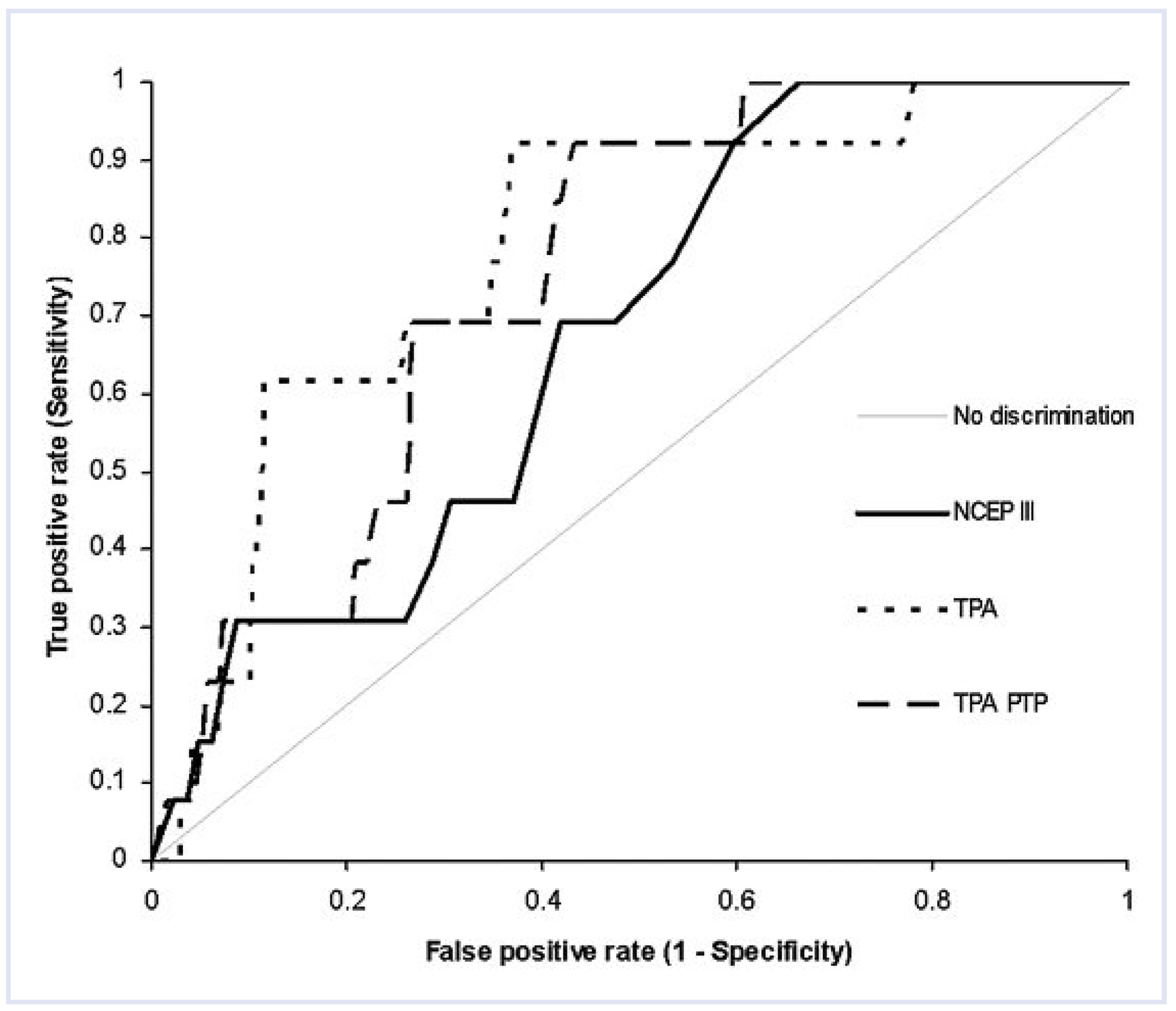
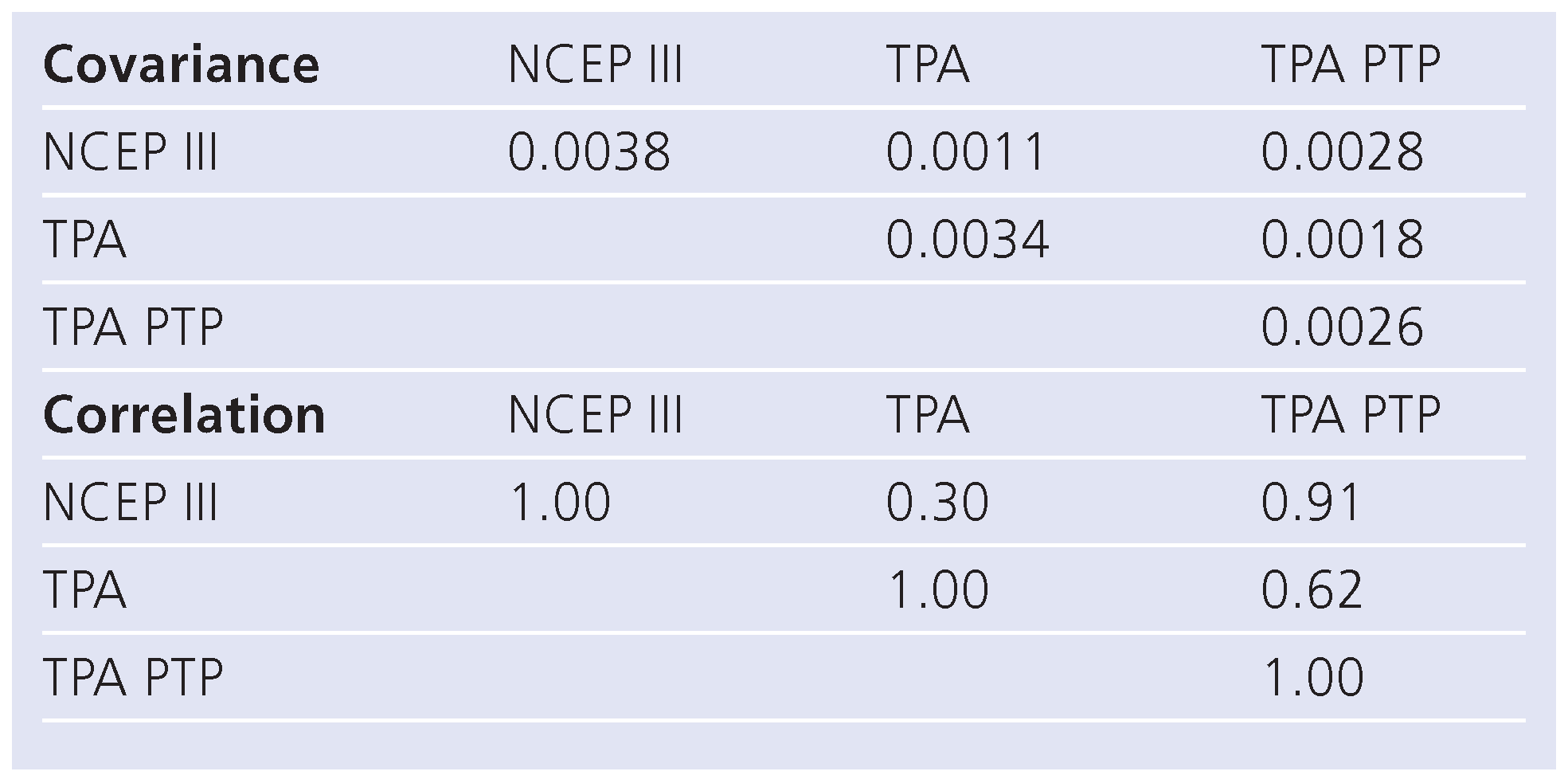
Correlations and diagnostic performance using ROC analysis of NCEP III, TPA, and TPA-PTP (Table 4 and Table 5, Figure 2)
Net reclassification improvement
Discussion
Limitations
Conclusions
Conflicts of Interest
References
- Assmann, G.; Cullen, P.; Schulte, H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) Study. Circulation 2002, 105, 310–5. [Google Scholar] [CrossRef] [PubMed]
- European guidelines on cardiovascular disease prevention in clinical practice. EJCPR 2003, 10 (Suppl 1), S1–78.
- Executive Summary of the third report of the national cholesterol education program. NCEP Adult Treatment Panel III. JAMA 2001, 285, 2486–97. [Google Scholar]
- Albert, M.; Glynn, R.; Ridker, P. Plasma Concentration of C-Reactive Protein and the Calculated Framingham Coronary Heart Disease Risk Score. Circulation 2003, 108, 161–5. [Google Scholar] [CrossRef] [PubMed]
- ACCF/AHA 2007 Clinical Expert Consensus Document on Coronary Artery Calcium Scoring by Computed Tomography in Global Cardiovascular Risk Assessment and in Evaluation of Patients With Chest Pain. Circulation 2007, 115, 402–26. [CrossRef] [PubMed]
- Lorenz, M.; Markus, H.; Bots, M.; Rosvall, M.; Sitzer, M. Prediction of Clinical Cardiovascular Events With Carotid Intima-Media Thickness. A Systematic Review and Meta-Analysis. Circulation 2007, 115, 459–67. [Google Scholar] [CrossRef] [PubMed]
- Obuchowski, N.A. Receiver operating characteristic curves and their use in radiology. Radiol 2003, 229, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Cook, N. Use and Misuse of the Receiver Operating Characteristic Curve in Risk Prediction. Circulation 2007, 115, 928–35. [Google Scholar] [CrossRef] [PubMed]
- Bayes, R.T. An Essay Toward Solving a Problem in the Doctrine of Chances. Philos. Trans. R. Soc. London 1958, 53, 370–418. [Google Scholar] [CrossRef]
- Spence, D.; Eliasziw, M.; DiCicco, M.; et al. Carotid plaque area: a tool for targeting and evaluation of vascular preventive therapy. Stroke 2002, 33, 2916–22. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.; D’Agostino, R.; D’Agostino, R.B., Jr.; Vasan, R. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008, 27, 157–72. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, S.; Melander, O.; Anevski, D.; et al. Polymorphisms Associated with Cholesterol and Risk of Cardiovascular Events. N Engl J Med 2008, 358, 1240–9. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.; DeLong, D.; Pearson, C. Comparing the areas under two or more correlated receiver operating curves. A nonparametric approach. Biometrics 1988, 44, 837–45. [Google Scholar] [CrossRef] [PubMed]
- Romanens, M.; Ackermann, F.; Spence, D.; et al. Improvement of cardiovascular risk prediction: Time to review current knowledge, debates, and fundamentals on how to assess test characteristics. European Journal of Cardiovascular Prevention & Rehabilitation 2010, 17, 18–23. [Google Scholar]

© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Romanens, M.; Ackermann, F.; Schwenkglenks, M.; Szucs, T.; Spence, J.D. Posterior Probabilities in Sequential Testing Improve Clinical Cardiovascular Risk Prediction Using Carotid Total Plaque Area and C-Statistics. Cardiovasc. Med. 2011, 14, 53. https://doi.org/10.4414/cvm.2011.01565
Romanens M, Ackermann F, Schwenkglenks M, Szucs T, Spence JD. Posterior Probabilities in Sequential Testing Improve Clinical Cardiovascular Risk Prediction Using Carotid Total Plaque Area and C-Statistics. Cardiovascular Medicine. 2011; 14(2):53. https://doi.org/10.4414/cvm.2011.01565
Chicago/Turabian StyleRomanens, Michel, Franz Ackermann, Matthias Schwenkglenks, Thomas Szucs, and J. David Spence. 2011. "Posterior Probabilities in Sequential Testing Improve Clinical Cardiovascular Risk Prediction Using Carotid Total Plaque Area and C-Statistics" Cardiovascular Medicine 14, no. 2: 53. https://doi.org/10.4414/cvm.2011.01565
APA StyleRomanens, M., Ackermann, F., Schwenkglenks, M., Szucs, T., & Spence, J. D. (2011). Posterior Probabilities in Sequential Testing Improve Clinical Cardiovascular Risk Prediction Using Carotid Total Plaque Area and C-Statistics. Cardiovascular Medicine, 14(2), 53. https://doi.org/10.4414/cvm.2011.01565




