Introduction
Heart injuries with different aetiologies are associated with cardiac tissue inflammation and usually result in a common final pathway of cardiac pathological remodelling and fibrosis, promoting the development of heart failure. Cardiac inflammation, myocarditis, plays an important role in these processes. Myocarditis is defined as a presence of inflammatory infiltrates in the myocardium with necrosis and/or degeneration of adjacent cardiomyocytes [
1]. About 10–20% of patients with histological evidence of myocardial inflammation develop symptoms of inflammatory dilated cardiomyopathy (iDCM) [
2]. iDCM represents up to 50% of all cases of dilated cardiomyopathy, and represents the most common cause of heart failure in young patients, who are frequently less than 40 years old.
In humans, iDCM most often results from infections with viruses, such as coxsackievirus B3, adenoviruses or parvovirus B19, or protozoan
Trypanosoma cruzi (Chagas disease) [
3], and is often associated with autoimmune responses against heart tissue antigens. Viral infections and the resulting immune response induce massive mononuclear cell infiltration at the injury site. Cardiac tissue is infiltrated by hematopoietic cells including granulocytes, monocytes, macrophages, dendritic cells, mast cells, T and B lymphocytes and cell progenitors. Cells infiltrating the myocardium persist for days at the injury site and contribute to inflammation, proteolysis, phagocytosis, angiogenesis, and play a pivotal role in tissue remodelling. Inflammatory cells release various cytokines such as interleukin-1, -6, -10, -12, -17, interferon-γ, transforming growth factor-β, and tumour necrosis factor-α, chemokines, and matrix metalloproteinases and their tissue inhibitors [
4,
5], and thus promote pathological processes.
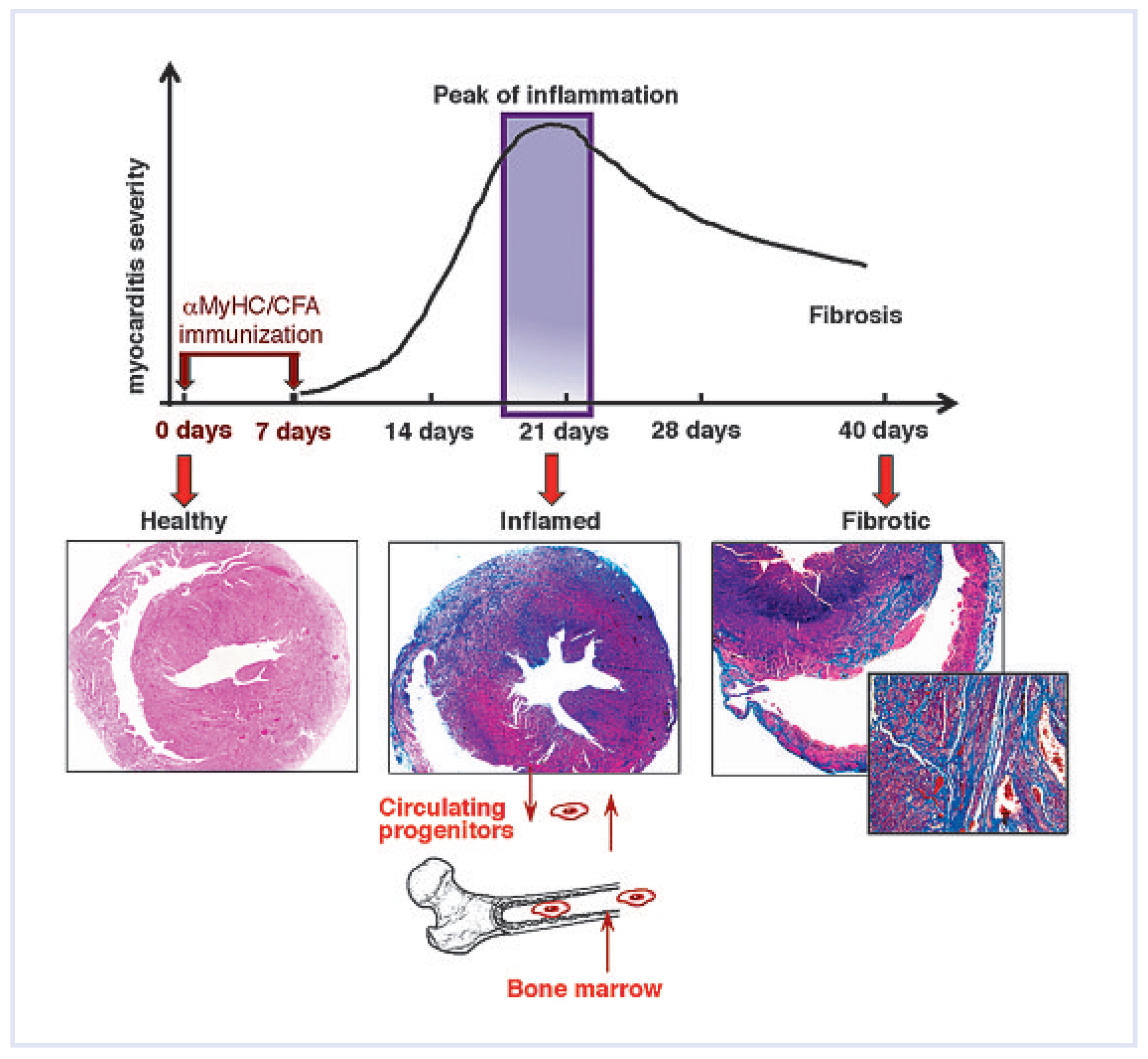
In homeostasis, the myocardial extracellular matrix represents a highly organised, dynamic network of fibrillar collagen, proteoglycans and glycoproteins. Heart tissue injury often alerts physiological extracellular matrix turnover and normal myocardial structure. Instead, the pathological tissue-remodelling process is characterised by a disproportionate accumulation of mainly type I collagen and collagen-producing fibroblasts progresses, which finally results in cardiac fibrosis [
6,
7]. Enhanced fibrosis leads to stiffening of the ventricles and impaired diastolic filling that indicates the irreversible end stage of the heart disease. In myocarditis-triggered iDCM, fibrosis results as a consequence of massive cell infiltrations within the myocardium, however, it is rarely associated with a substantial loss of cardiomyocytes. Even so, the chronic inflammation promotes ongoing myocardial fibrosis, which is a hallmark of end-stage heart failure (
Figure 1).
Clinical manifestations of myocarditis are highly variable, varying from no symptoms to heart failure. Patients usually show a broad range of symptoms, for example chest pain, arrhythmias, embolic events, congestive heart failure, and sometimes cardiogenic shock [
8]. The proposed classification of myocarditis based on the analysis of the histological findings of biopsies and the clinical course of the patients [
9]. Accordingly, the patients may suffer from (1.) fulminant myocarditis reflecting severe cardiovascular insufficiency with histological evidence of active myocarditis, and left ventricular dysfunction, (2.) acute myocarditis with established ventricular dysfunction and histological evidence of active myocarditis (this form of disease may progress to iDCM), (3.) chronic active myocarditis resulting in left ventricular dysfunction and progressing to myocardial fibrosis and iDCM, (4.) chronic persistent myocarditis characterised by continuing cardiovascular symptoms and histological signs of myocyte necrosis but without significant ventricular pathological changes, (5.) giant cell myocarditis, reflecting a poor outcome and high probability of death, and (6.) eosinophilic myocarditis also characterised with a poor prognosis. Almost all these forms of myocarditis can be currently reproduced in animal, mouse or rat, experimental models giving the opportunity to study the pathogenesis of this variable disease.
Cardiac stem cells—can they rescue failing myocardium?
The discovery in the adult mammalian myocardium of a population of resident cardiac stem cells (CSCs) with the potential to differentiate into cardiomyocytes [
10,
11,
12] was a milestone in cardiovascular biology. CSCs were identified in the healthy myocardium in their specific niches, which become activated following tissue damage, proliferate and replace the apoptotic or necrotic cells, for example cardiomyocytes, smooth muscles, or endothelial cells [
13]. It has been also postulated that CSCs sustain the cardiomyocytes turnover [
11,
14]. However, the lack of substantial myocardial regeneration following heart injury strengthened the requirement for development of alternative methods supporting heart regeneration.
CSCs are not an exclusive cellular source of cardiomyocytes in the adult mammalian body. Much hope was placed in the bone-marrow-derived stem cells due to easy access, standardised procedures, autologous origin and minimal risk of carcinogenesis. Indeed, hematopoietic and mesenchymal stem cells were successfully differentiated into cardiac lineage, and were shown to some extent to regenerate the injured myocardium [
15,
16,
17]. Furthermore, we demonstrated that the healthy mouse heart contains 1–2% CD45+ cells originating from bone marrow [
18]. We found that 1– 2% of these bone-marrow-derived cells express CD133 antigen, mouse homolog of human AC133, which is a well described marker of hematopoietic, embryonic, and adult progenitor cells. Heart-resident CD133-expressing cells can be successfully differentiated into cardiomyocytes in vitro and in vivo.
However, encouraging results with the use of hematopoietic cells were confronted by other studies showing that the cardiac differentiation in vivo occurs in most cases via cell fusion [
19], and the majority of stem and progenitor cells transplanted into the damaged myocardium adopt hematopoietic rather than cardiac phenotypes [
20]. Probably due to these reasons, nearly all stem-cell-based clinical trials revealed low efficacy and poor prognosis for the future [
21]. Although the role of CSCs and bone-marrow-derived stem cells in inflammatory heart diseases remains largely speculative, several lines of evidence point to their beneficial paracrine effects, rather than a cellular source for direct myocardial regeneration (cardiomyocytes replacement).
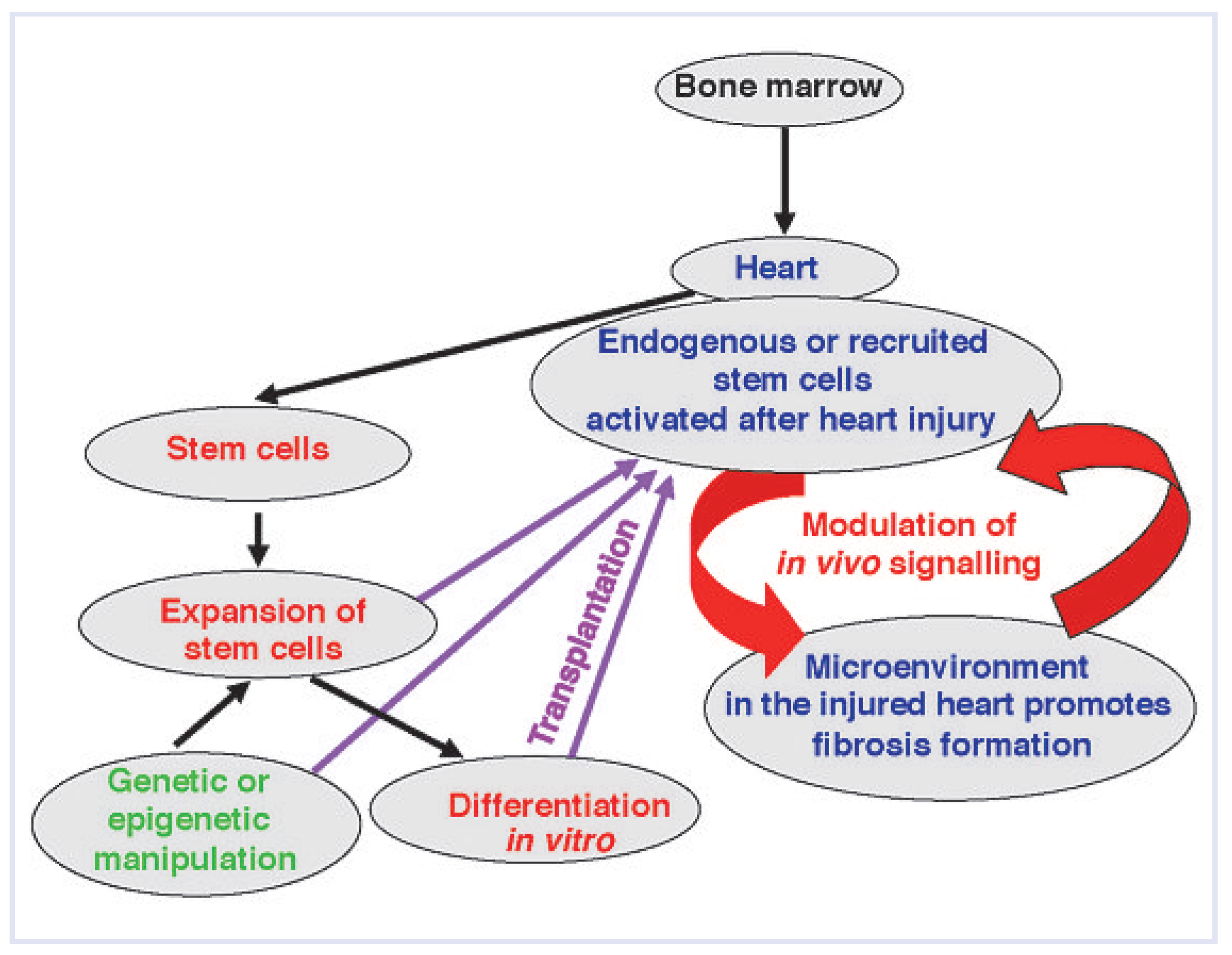
In conclusion, despite the existence of either endogenous or exogenous stem and progenitor cells with the cardio-regenerative potentials, heart injury generally ends up as heart failure. Therefore, the question arises as to why these stem and progenitor cells fail to repair the damaged myocardium.
The fate of stem cells infiltrating the heart—the decisive role of the myocardial microenvironment
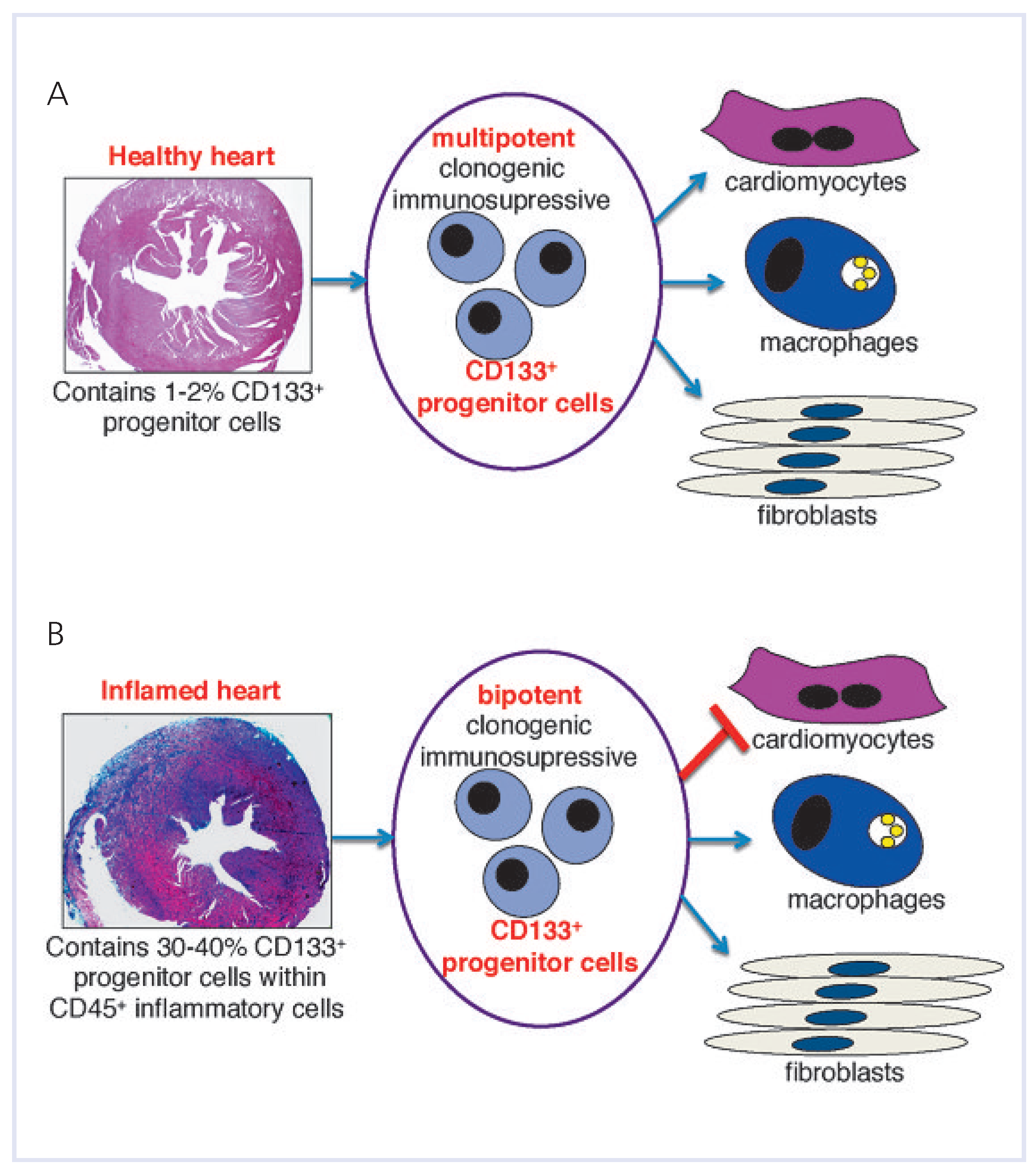
In response to myocardial injury, stem and progenitor cells are recruited from the bone marrow and blood into the injury sites, and endogenous CSCs are activated, proliferate rapidly and regenerate the injured myocardium. In inflammatory heart disorders, bone-marrow-derived cell progenitors, rather than CSCs play a pivotal role in the disease development and in further pathological progression. We used a mouse model of experimental autoimmune myocarditis (EAM) to study the progression of acute cardiac inflammation into myocardial fibrosis and end-stage heart failures. In the EAM, heart-infiltrating cells contain a pool of CD133-expressing progenitors of bone marrow origin. These cells represent the cellular source for monocytes during the acute inflammatory phase and for pathological myofibroblasts at the late, chronic stage of the disease [
22,
23] (
Figure 2). Conversion of the CD133+ progenitors into myofibroblasts is TGF-β-dependent. Accordingly, TGF-β blockade suppresses post-inflammatory fibrosis development in the EAM model [
23]. In the context of other inflammatory models, recent data suggest that monocyte precursor cells can differentiate into diverse subpopulations of inflammatory cells, such as dendritic cells and macrophages, depending on the cytokine microenvironment [
24,
25,
26]. Furthermore, the insights from ischaemic heart disease models suggests that bone-marrow-derived progenitor cells recruited to the inflamed heart represent a potential cellular source for fibrosis as well [
27]. In the light of recent data, it now becomes evident that a delicate balance of pro-inflammatory and profibrotic cytokines determines the fate of heart-infiltrating progenitors and thus directly influences the morphological and functional phenotype of the affected heart. To summarise, it is assumed that iDCM progression in inflammatory heart disorders critically depends on the fate of inflammatory progenitor cells. The inflammatory cytokine balance creating specific myocardial signalling milieus is decisive in directing the fate of the inflammatory progenitors. These statements are in accordance with outcomes from other inflammatory diseases models, and emphasise the crucial role of the cytokine environment dictating the differentiation fate of tissue-recruited progenitors [
26,
28]. Therefore, the influence of the myocardial microenvironment on the function and fate of transplanted/recruited progenitor cells in iDCM seems to be crucial for the development of effective cell-based therapies.
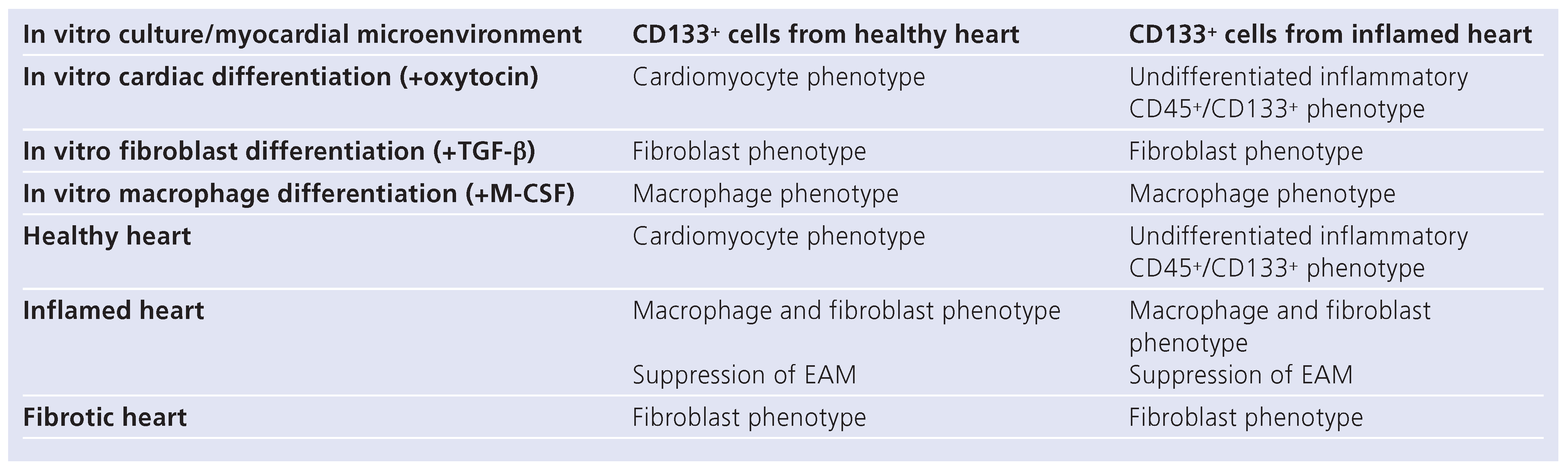
The use of hematopoietic stem cells in heart disorders remains controversial. On the one hand, some enthusiastic reports describe the cardio-regenerative potential of bone-marrow-derived tissue-resident cells [
13,
15]. On the other hand, the majority of the studies evaluating bone-marrow-derived stem cell therapies showed minimal in vivo cardiomyocyte differentiation [
27], or even question such cell-based therapies at all [
20,
29]. The critical role of the microenvironment in determining the fate of precursor cells may explain these contradictory findings. Herein, I want to highlight the multi-lineage nature of stem cells (particularly of hematopoietic origin). Thus, not only cellular substrate matters, but also triggering a proper differentiation program. It is difficult to unequivocally estimate the contribution of genetic and epigenetic (the role of microenvironment) determinants in this process. Possibly it is highly dependent on the specific stem cell population and disease characteristics. In this context, our findings demonstrate that CD133 expression defines a specific population of heart-infiltrating bone-marrow-derived progenitor cells that differentiated into fibroblasts, thereby promoting tissue fibrosis during the late stages of iDCM, point to careful evaluation of stem and progenitor cells isolated from injured organs for use in regenerative medicine. Accordingly, we have compared the characteristic, in vitro and in vivo differentiation potentials and anti-inflammatory properties of CD133-expressing cells either derived from the healthy or inflamed hearts (
Table 1). These data illustrated that despite the phenotypical similarity of CD133+ progenitors expanded from healthy hearts [
18] (
Figure 3A) and isolated from inflamed myocardium [
23] (
Figure 3B), both populations display different abilities to become cardiomyocytes (distinct regenerative capacity). This suggests that the microenvironment of the inflamed heart disables the expansion of progenitor cells with the regenerative capacity (capability to become cardiomyocytes).
As stated above, the hostile microenvironment affects the function of inflammatory stem and progenitor cells, and possibly also resident CSCs. It is expected that excessive inflammation promotes angiogenesis and affects survival of resident cells, such as endothelial cells, cardiomyocytes, as well as inflammatory cells in the myocardium. Hence, current evidence supports the view that the specific microenvironment of the affected myocardium prevents the cardio-regenerative potential of stem cells in the inflamed heart. Instead, this non-physiological microenvironment promotes generation of monocytes and myofibroblasts [
23,
30]. These results are in line with studies on infracted [
31] or hypertrophic [
27] hearts showing that bone-marrowderived progenitor cells actively participate in scar formation after myocardial injury. Our studies support the critical role of the cardiac microenvironment on the fate of transplanted stem cells. We have demonstrated that CD133+ progenitors administrated into the myocardium at different stages of EAM acquired different cellular phenotypes [
18,
23]. Diverse stimulants, such as cytokines, chemokines, apoptotic and necrotic cells, and extracellular matrixes are differently present in the healthy, inflamed and fibrotic myocardium, and create a unique myocardial signalling milieu. Apparently, the microenvironment of the inflamed and fibrotic heart promotes the fibroblast fate of inflammatory progenitors. Therefore, reduction or completely reverting of the naturally occurring the fibroblast fate of inflammatory progenitor cells in iDCM still remains a challenge.
Stem-cell-based therapies
Stem-cell-based therapies include transplantation of exogenous stem cells or stem-cell-derived committed or terminally differentiated cells, or specific activation of endogenous stem cells. For treatment against ischaemic and non-ischaemic heart disorders, different types of somatic stem and progenitor cells including hematopoietic stem cells, mesenchymal stem cells, epithelial progenitor cells, CSCs, adipose-tissue-derived stem cells, skeletal myoblasts, and spermatogonial stem cells have been tested to improve cardiac function. Truly pluripotent stem cells, such as embryonic stem cells and induced pluripotent stem cells (iPS, reprogrammed skin fibroblasts) represent an excellent source for the generation of large numbers of cardiomyocytes. However, these cells are at increased risk of teratoma formation and are characterised by poor engraftment capability after transplantation. These limitations border their use for regenerative cell therapies at the moment. Transplantation of allogenic cells often results in immune rejection. In contrast to embryonic stem cells, iPS and CSCs represent sources of plurior multipotent stem cells that allow generating patientspecific cardiomyocytes for autologous transplantation. The minimal risk of teratoma formation together with the natural capacity for cardiomyocyte differentiation is an advantage of CSCs that become the natural candidate for stem-cell-based therapy in cardiovascular disorders. However, lack of definitive CSC-specific markers and standardised protocols for the isolation and expansion of these cells limits their therapeutic use. Thus, there are only few experimental data employing the use of CSCs as a cell source for heart repair [
17,
32,
33]. In this context, hematopoietic and mesenchymal stem cells represent a better cellular source for cell interventions because of their accessibility. Furthermore, the contribution of bone-marrow-derived stem and progenitor cells to cardiac repair and heart function recovery may occur in multiple fashions. This includes differentiation into cardiomyocytes, smooth muscle and endothelial cells, fusion of stem or progenitor cells with host recipient cardiac cells or action through paracrine effects affecting the neovascularisation, inflammation, wound healing and possibly activation of the resident stem and progenitor cells [
13].
Current clinical randomised clinical trials with acute myocardial infarction or ischaemic heart failure show that bone marrow cell therapy improves myocardial perfusion and contractile performance in patients with acute myocardial infarction, heart failure and chronic myocardial ischaemia [
21]. Nevertheless, all these studies showed only transient improvement (up to 6 months) of left ventricular ejection fraction, and no longer statistically significant upgrading after 18 months. Early clinical trials have provided a signal that stem cell therapy can enhance tissue perfusion and contractile performance of the injured human heart [
21]. However, the current knowledge supports the concept that stem cells act in the paracrine manner rather than contributing directly to the cardiomyocytes regeneration. The concept that endogenous or exogenously transplanted stem cells promote this functional adjustment and heart repair after myocardial damage brings new understanding of the stem-cell-based therapies in cardiovascular diseases. Unfortunately, there are almost no reports presenting the clinical trial with the use of stem cells in iDCM patients. Data from animal models demonstrate that the status of the myocardium is critical for the success of stem cell administration. For example, transplantation of stem cells during the chronic phase of the disease may even accelerate disease progression. Therefore, further studies in animal models of inflammatory heart diseases are necessary. The ultimate outcome of such studies will convey the important knowledge translatable into other cardiovascular injuries. Of note, most ischaemic heart injuries are associated with a transient and often local tissue inflammation. Therefore, the iDCM models are rather “macro” models for studying the pathological inflammatory and post-inflammatory processes. The mechanisms can be extended to ischaemic heart injuries or myocardial infarction representing “micro” models in terms of the inflammatory response.
Published reports have shown that exogenously ad-ministrated stem cells of different origins efficiently abrogate the autoimmune myocarditis development and further heart failure progression in animal models of iDCM [
18,
34,
35,
36]. It seems that the protective function of stem cells depends on nitric oxide-mediated suppression of heart-specific CD4+ T-cell expansion [
18,
22]. Furthermore, several studies showed that mesenchymal stem cell transplantation prevents heart failure development and improves cardiac function by inducing neovascularisation and by inhibiting inflammatory cytokine production [
34,
36]. These data highlighted appropriate timing of stem or progenitor cells injections for the beneficial effect [
18,
35]. However, such treatment requires the in vitro culture of stem cells that always increase the risk of potential side effects.
The development of novel clinically relevant stemcell-based therapies aiming at cardiac tissue regeneration requires additional handling. It includes ex vivo manipulation of progenitor cells rendering them resistant to the pathogenic environment, the combined in vivo targeting of specific inflammatory modulators inhibiting the naturally occurring fibroblast differentiation, and if possible, promoting the cardio-regenerative capacity of progenitor cells (
Figure 4). In fact, ex vivo pretreatment of autologous bone-marrow-derived progenitor cells with cardiomyogenic growth factors boosts their cardiac differentiation and their functional regenerative capacity in vivo [
37]. Changing the specific cytokine milieu defining the fate of inflammatory progenitors may give rise to a novel treatment strategy against chronic fibrotic processes in iDCM. Among various inflammatory cytokines produced in response to tissue injury, hematopoietic cytokines, naturally released after infection or inflammation, play an important role in the immune response. These cytokines act through the regulation of inflammatory cytokine balance and control tissue reparative processes through the recruitment, activation and differentiation of bone marrow cellular compartments [
38]. Recent experimental and clinical data suggest that hematopoietic cytokines are actively involved in the pathophysiology of several cardiovascular disorders, including vascular remodelling, atherosclerosis, arterial hypertension, acute coronary syndromes, ischaemia/reperfusion injury, post-infarction left ventricular remodelling and chronic heart failure [
38]. There is a growing body of evidence that these cytokines play a crucial role in the attenuation of myocardial inflammation and fibrosis following heart injury [
38]. It is therefore tempting to hypothesise that modulating the hematopoietic cytokine systems might attenuate myocardial fibrosis and heart failure progression following the heart inflammation. Treatment with the hematopoietic cytokines might modulate the function of inflammatory progenitors by promoting the accumulation of monocytes within the myocardium, instead of pathological myofibroblasts. Accumulated monocytes silence inflammatory processes in a paracrine fashion (for example via nitric oxide production) and additionally attenuate further myocardial fibrosis formation. This hypothesis is in accordance with a previous report [
39] showing that CSCs act via autocrine mechanisms, secreting hepatocyte growth factor and insulin-like growth factor-1 that promote, on the one hand, the self-activation of CSCs, and on the other hand, reconstitution of dead myocardium and recovery of cardiac function.