How to Transform You into a Radialist: Tips and Tricks
Summary
Five rules to transform you into a radialist
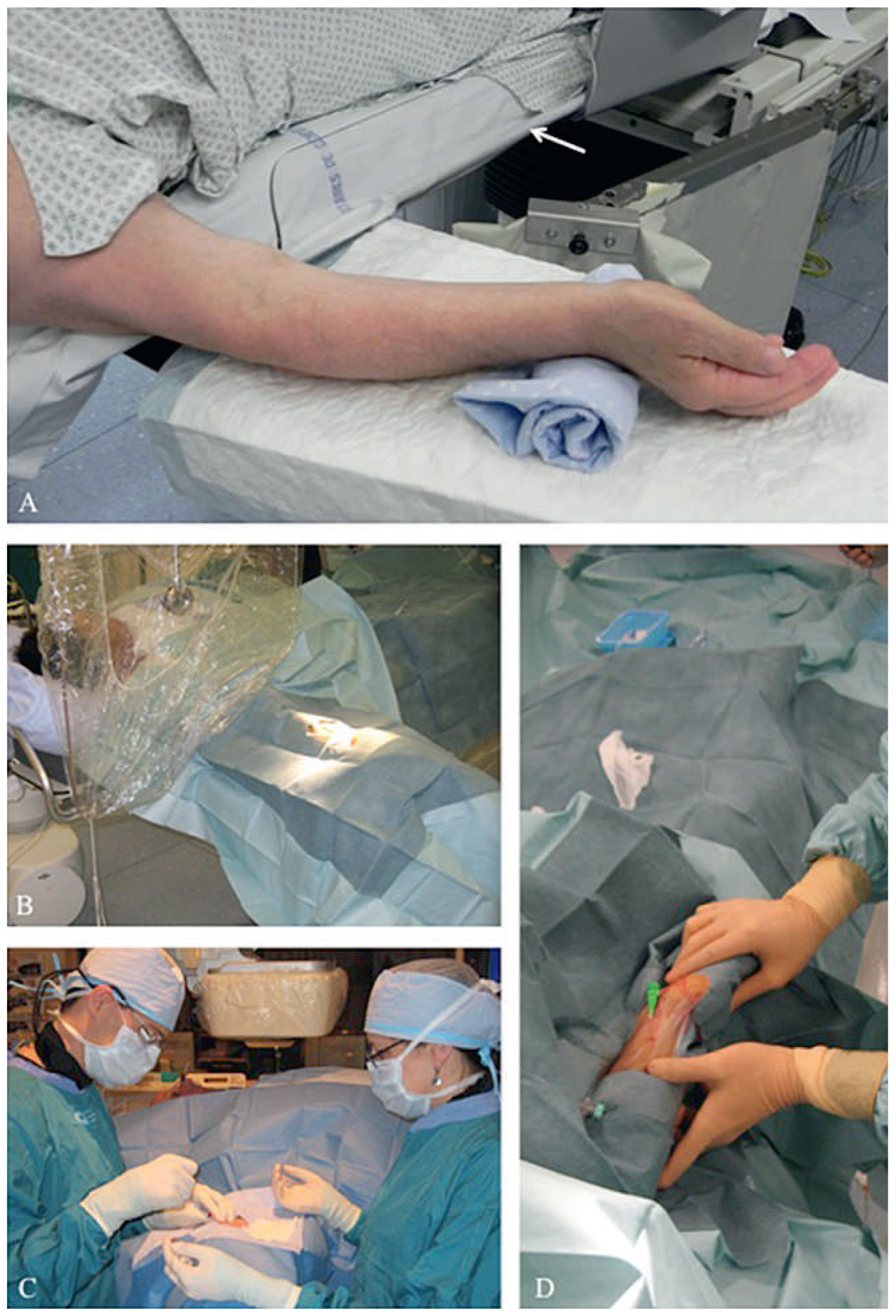
Rule N°1: be comfortable for radial artery puncture
Arm setup
Needle selection and techniques for puncture
Rule N°2: make the patient comfortable
Premedication
Arm support
Local anaesthesia
Sheath size
Prevention of radial artery spasm
Prevention of radial artery occlusion (RAo)
 |
Rule N°3: use the right wire
Resistance advancing the wire or catheter.
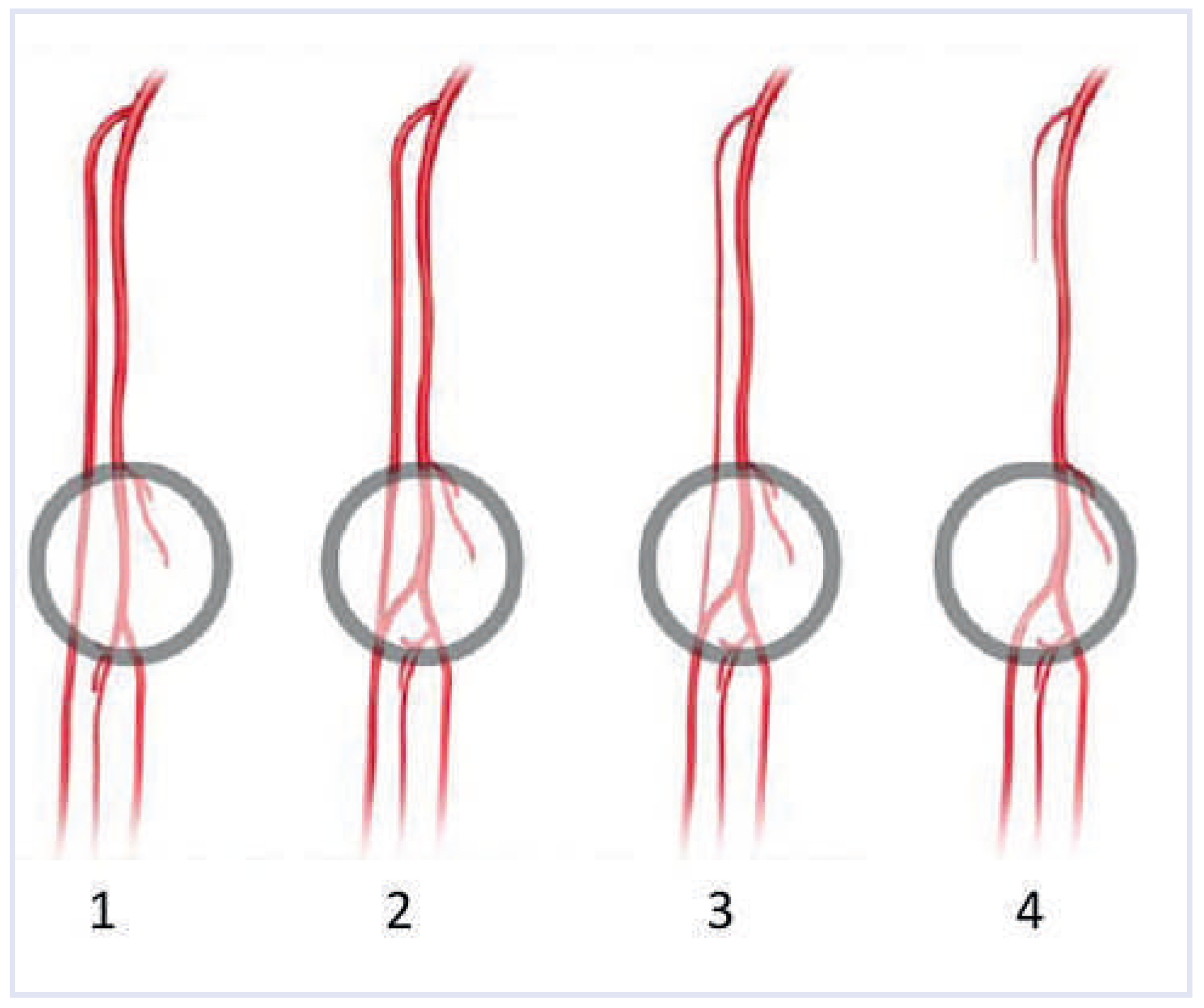
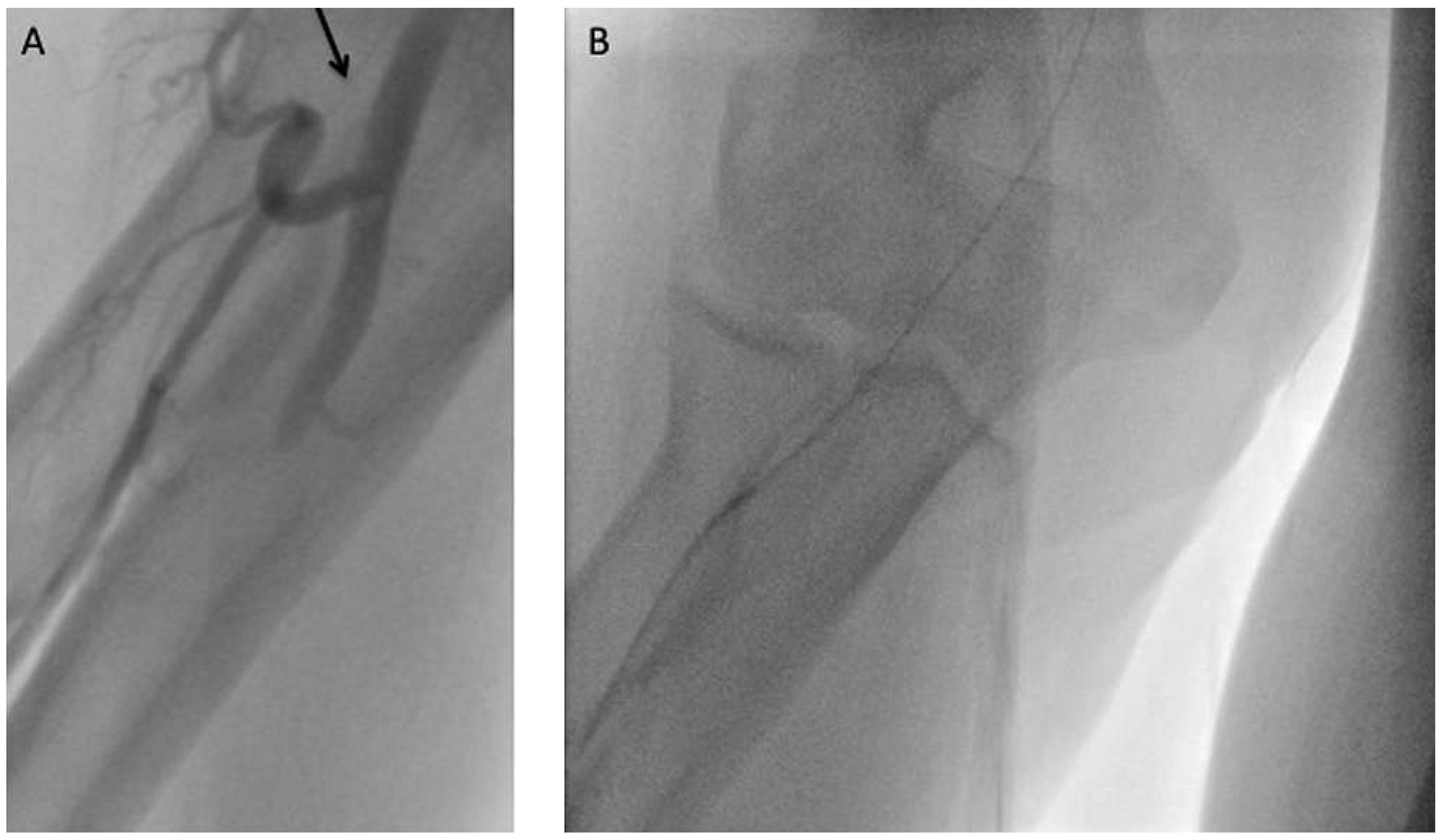
Radial artery tortuosity or loop
Subclavian artery tortuosity or loop
Exchanging catheters
Use of respiration
Rule N°4: choose the right catheter
Routine diagnostic catheter selection
Routine guide catheter selection
Rule N° 5: limit compression time
 |
How to manage specific situations Radial artery spasm
RA spasm during the procedure
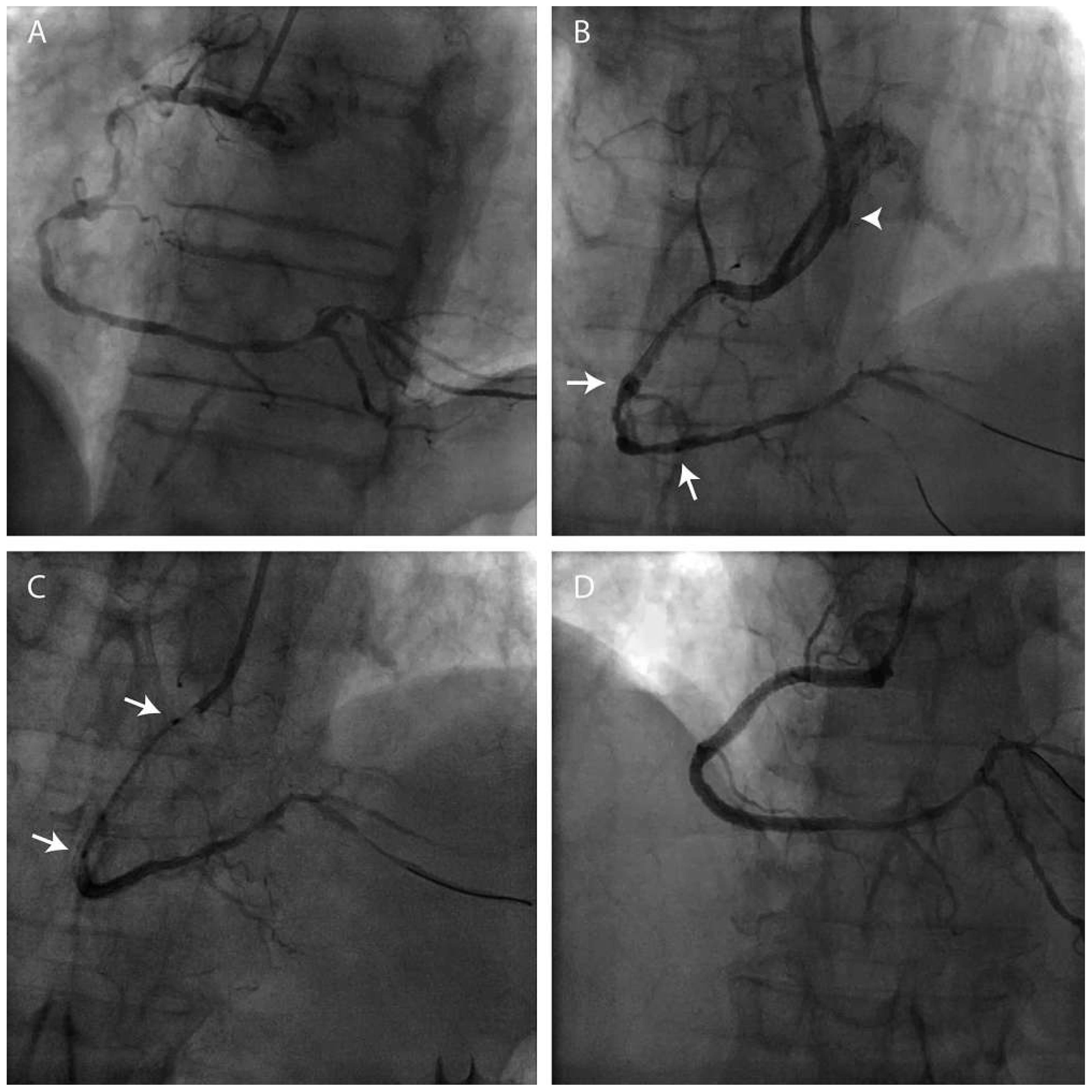
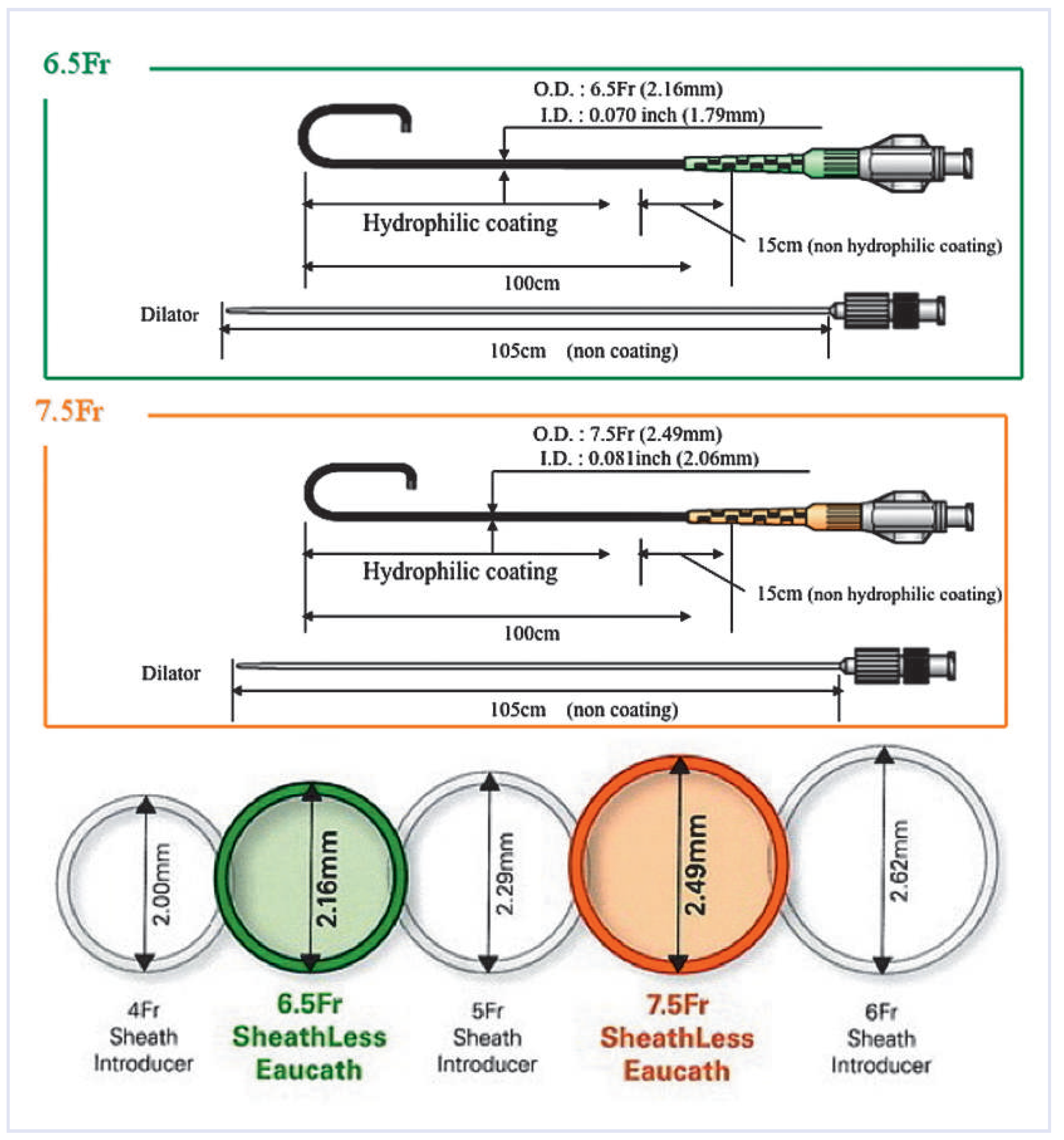
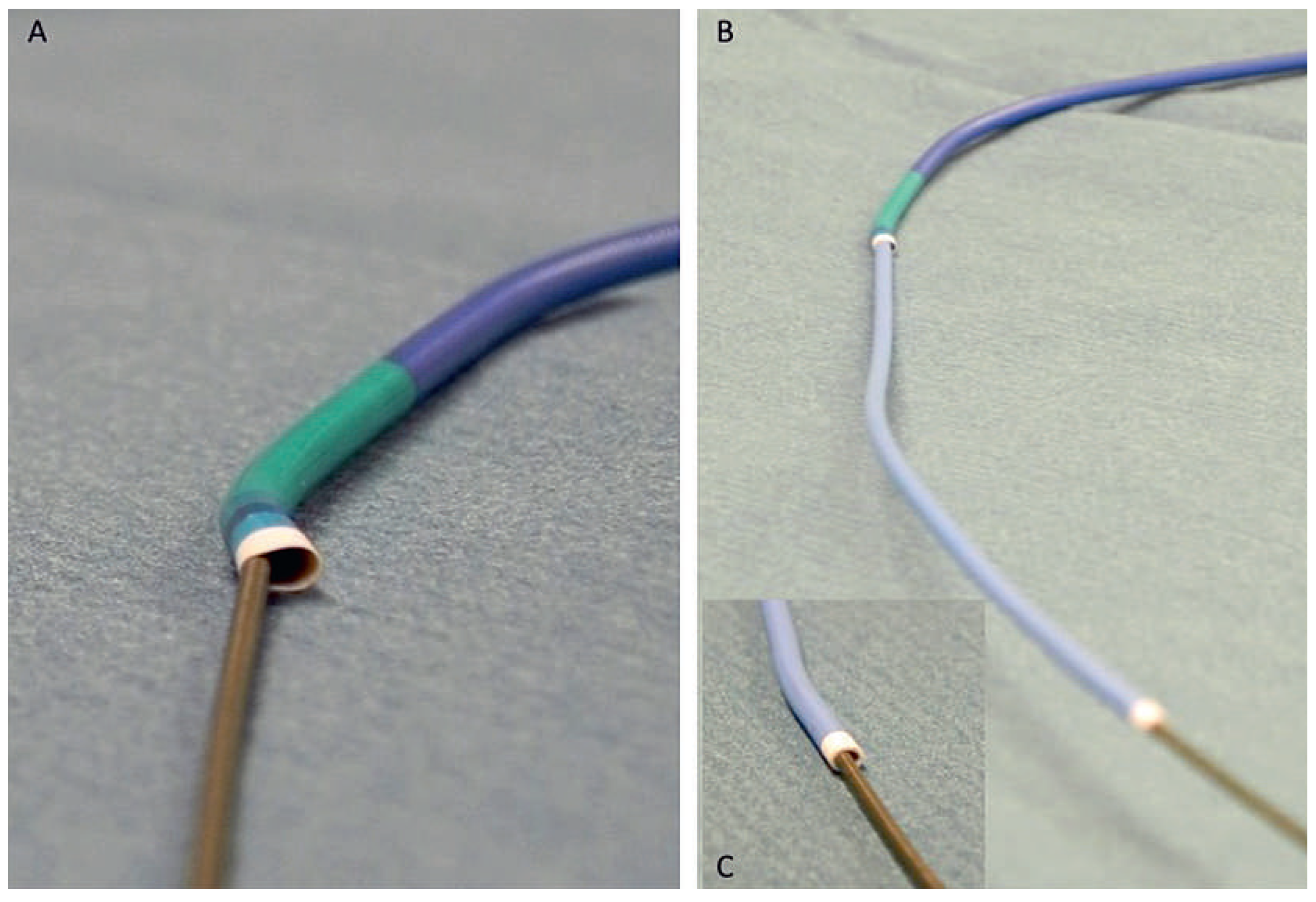
Sheathless catheter
Telescopic technique (mother and child technique).
Extreme spasm
Small calibre radial artery
Patients with coronary artery bypass grafts (CABG)
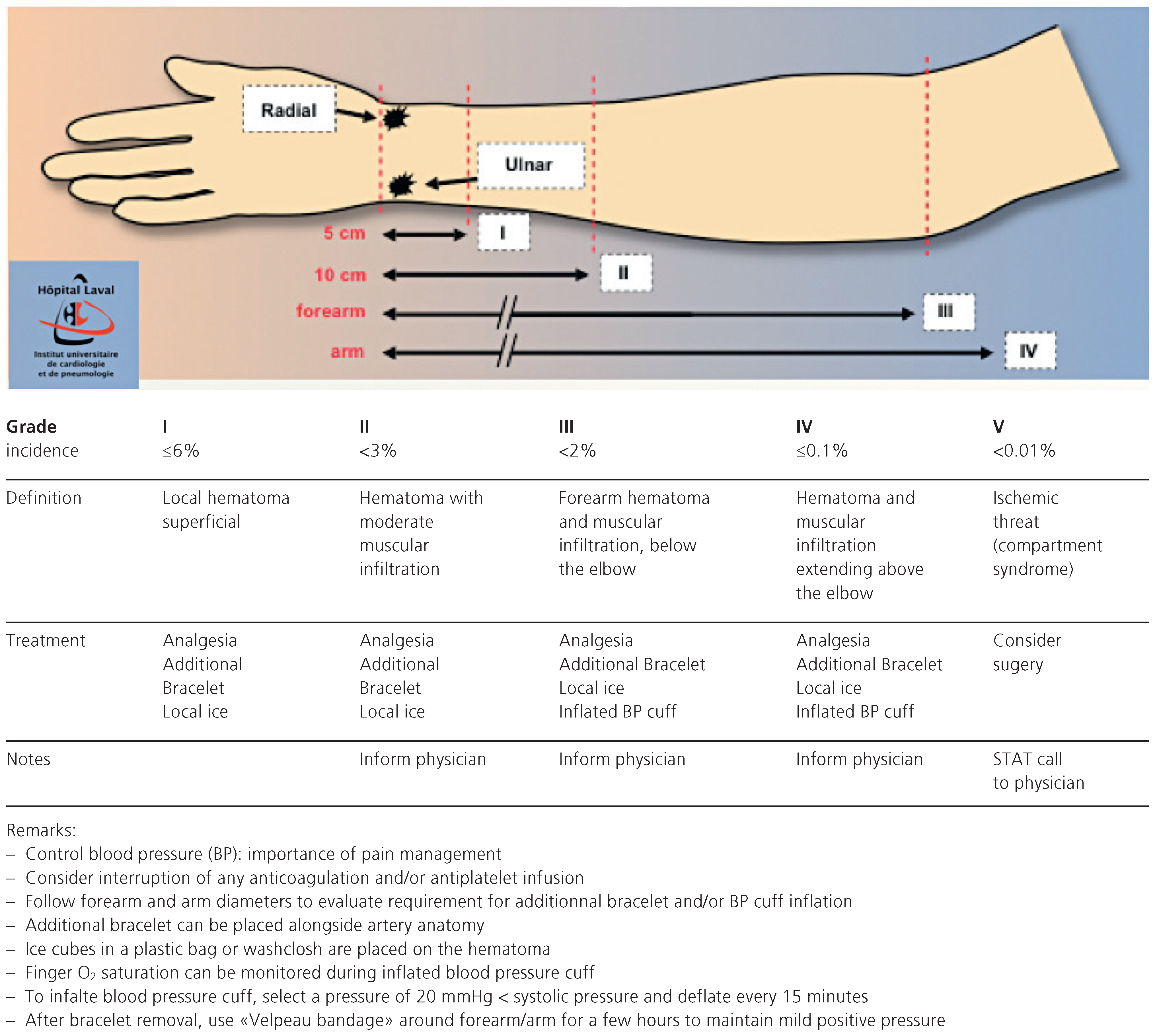
Local bleeding and haematoma
Left transradial approach
Conclusion
Funding
Conflicts of Interest
References
- Frangos, C.; Noble, S. How to transform you into a radialist: Literature review. Part I. Cardiovascular Medicine. 2011, 14, 277–82. [Google Scholar]
- Bertrand, O.F.; Rao, S.V.; Pancholy, S.; et al. Transradial approach for coronary angiography and interventions. Results of the first international transradial practice survey. J Am Coll Cardiol Intv. 2010, 3, 1022–31. [Google Scholar] [CrossRef] [PubMed]
- Pancholy, S.B.; Coppola, J.; Patel, T. Subcutaneous administration of nitroglycerin to facilitate radial artery cannulation. Catheter Cardiovasc Interv. 2006, 68, 389–91. [Google Scholar] [PubMed]
- Bertrand, O.F.; Larose, E.; Rodes-Cabau, J. Sub-cutaneous nitroglycerin: Good example of the “KISS” rule! Letter to the Editor Catheter Cardiovasc Interv. 2006, 70, 161. [Google Scholar] [CrossRef] [PubMed]
- Varenne, O.; Jégou, A.; Cohen, R.; et al. Prevention of arterial spasm dur-ing percutaneous coronary interventions through radial artery: The SPASM study. Cathet Cardiovasc Interv. 2006, 68, 231–5. [Google Scholar]
- Stella, P.R.; Kiemeneij, F.; Laarman, G.J.; et al. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Catheter Cardiovasc Diagn. 1997, 40, 156–8. [Google Scholar] [CrossRef]
- Spaulding, C.; Lefèvre, T.; Funk, F. Left radial approach for coronary an-giography: Results from a prospective study. Cathet Cardiovasc Diagn. 1996, 39, 365–70. [Google Scholar] [PubMed]
- Dahm, J.B.; Vogelgesang, D.; Hummel, A.; et al. A randomized trial of 5 vs. 6 French transradial percutaneous coronary interventions. Catheter Cardiovasc Interv. 2002, 57, 172–6. [Google Scholar] [CrossRef] [PubMed]
- Cubero, J.M.; Lombardo, J.; Perdrosa, C.; et al. Radial compression guided by mean artery pressure versus standard compression with a pneumatic device (RACOMAP). Catheter Cardiovasc Interv. 2009, 73, 467–72. [Google Scholar] [CrossRef] [PubMed]
- Velsecchi, O.; Vassileva, A.; Musumeci, G.; et al. Failure of transradial approach during coronary interventions : Anatomic considerations. Catheter Cardiovasc Interv. 2006, 67, 870–8. [Google Scholar] [CrossRef] [PubMed]
- Arzamendi, D.; Romeo, P.; Gosselin, G. Radial artery avulsion: A rare complication of percutaneous coronary intervention. Rev Esp Cardiol. 2011, 64, 62. [Google Scholar] [CrossRef]
- Pancholy, S.; Coppola, J.; Patel, T.; et al. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): A randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008, 72, 335–40. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.A.; Fath-Ordoubadi, F.; Fraser, D.G. Atraumatic complex transradial intervention using large bore sheathless guide catheter. Catheter Cardiovasc Interv. 2008, 72, 357–64. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.; D’Souza, S.; Hendry, C.; et al. Use of the sheathless guide catheter during transradial percutaneous coronary intervention: A feasibility study. Catheter Cardiovasc Interv. 2010, 75, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Trani, C.; Hamon, M.; et al. Transradial approach for coronary angiography and interventions in patients with coronary bypass grafts : Tips and tricks. Catheter Cardiovasc Interv. 2008, 72, 263–72. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, O.F.; De Larochellière, R.; Rodés-Cabau, J.; et al. A randomized study comparing same-day home discharge and abciximab bolus only to overnight hospitalization and abciximab bolus and infusion after transradial coronary stent implantation. Circulation. 2006, 114, 2636–43. [Google Scholar] [CrossRef] [PubMed]
- Sciahbasi, A.; Romagnoli, E.; Trani, C.; et al. Operator Radiation Exposure During Percutaneous Coronary Procedures Through the Left or Right Radial Approach: The TALENT Dosimetric Substudy. Circ Cardiovasc nterv. 2011, 4, 226–31. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Frangos, C.; Noble, S. How to Transform You into a Radialist: Tips and Tricks. Cardiovasc. Med. 2011, 14, 315. https://doi.org/10.4414/cvm.2011.01620
Frangos C, Noble S. How to Transform You into a Radialist: Tips and Tricks. Cardiovascular Medicine. 2011; 14(11):315. https://doi.org/10.4414/cvm.2011.01620
Chicago/Turabian StyleFrangos, Caroline, and Stéphane Noble. 2011. "How to Transform You into a Radialist: Tips and Tricks" Cardiovascular Medicine 14, no. 11: 315. https://doi.org/10.4414/cvm.2011.01620
APA StyleFrangos, C., & Noble, S. (2011). How to Transform You into a Radialist: Tips and Tricks. Cardiovascular Medicine, 14(11), 315. https://doi.org/10.4414/cvm.2011.01620




