Muscular Ventricular Septal Defect After Mitral and Aortic Valve Replacement
Abstract
Introduction to Case Series
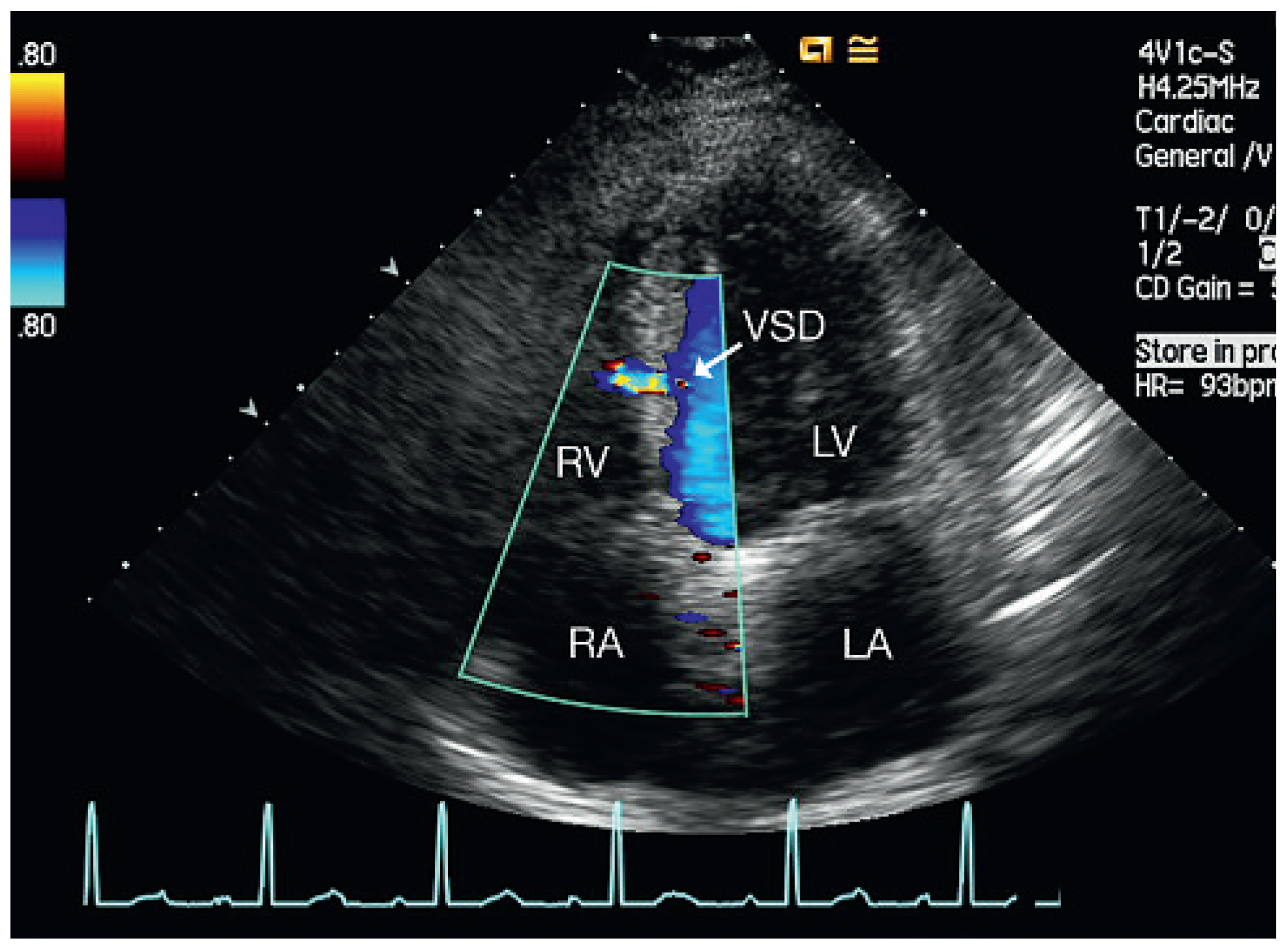
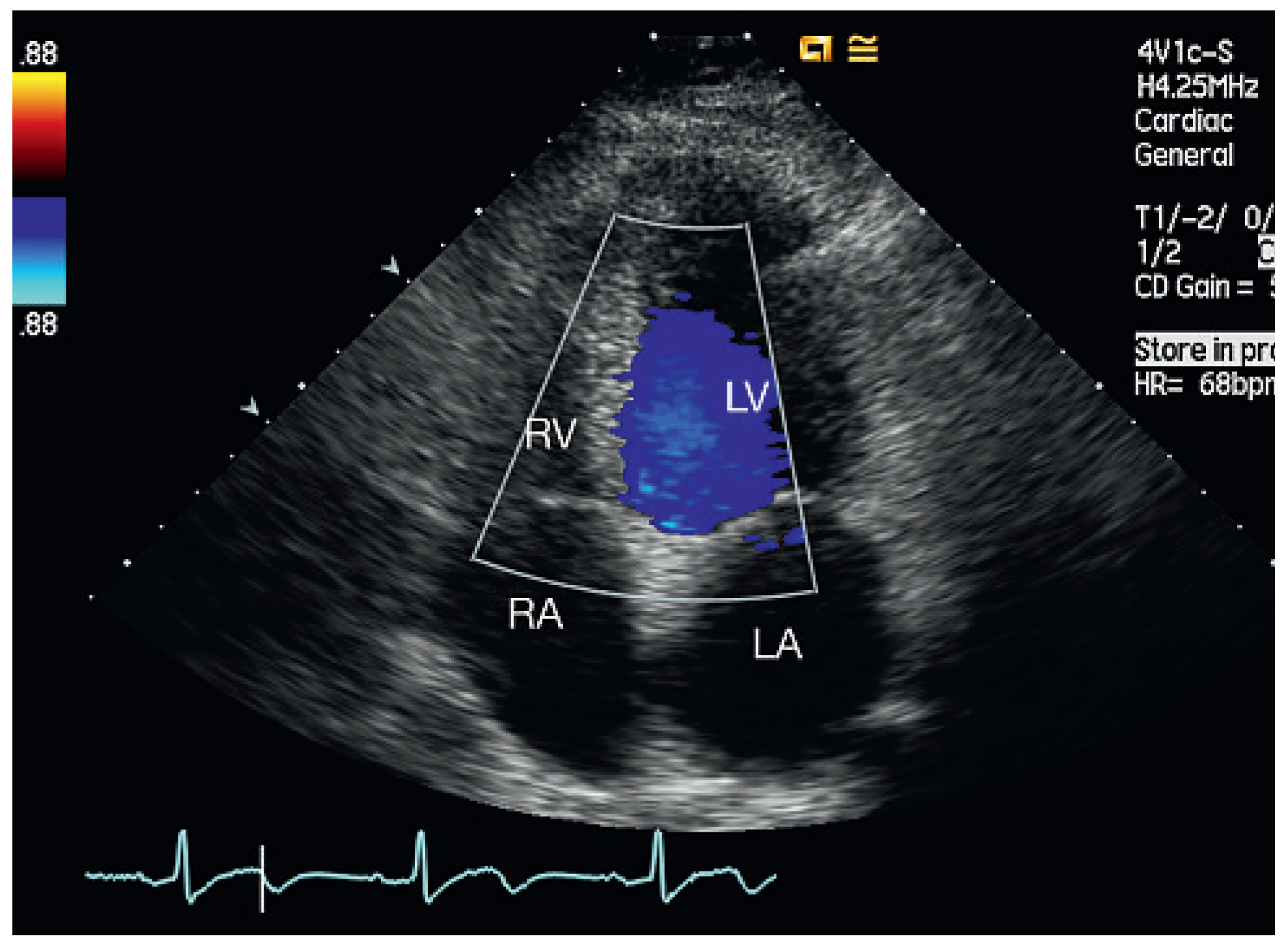


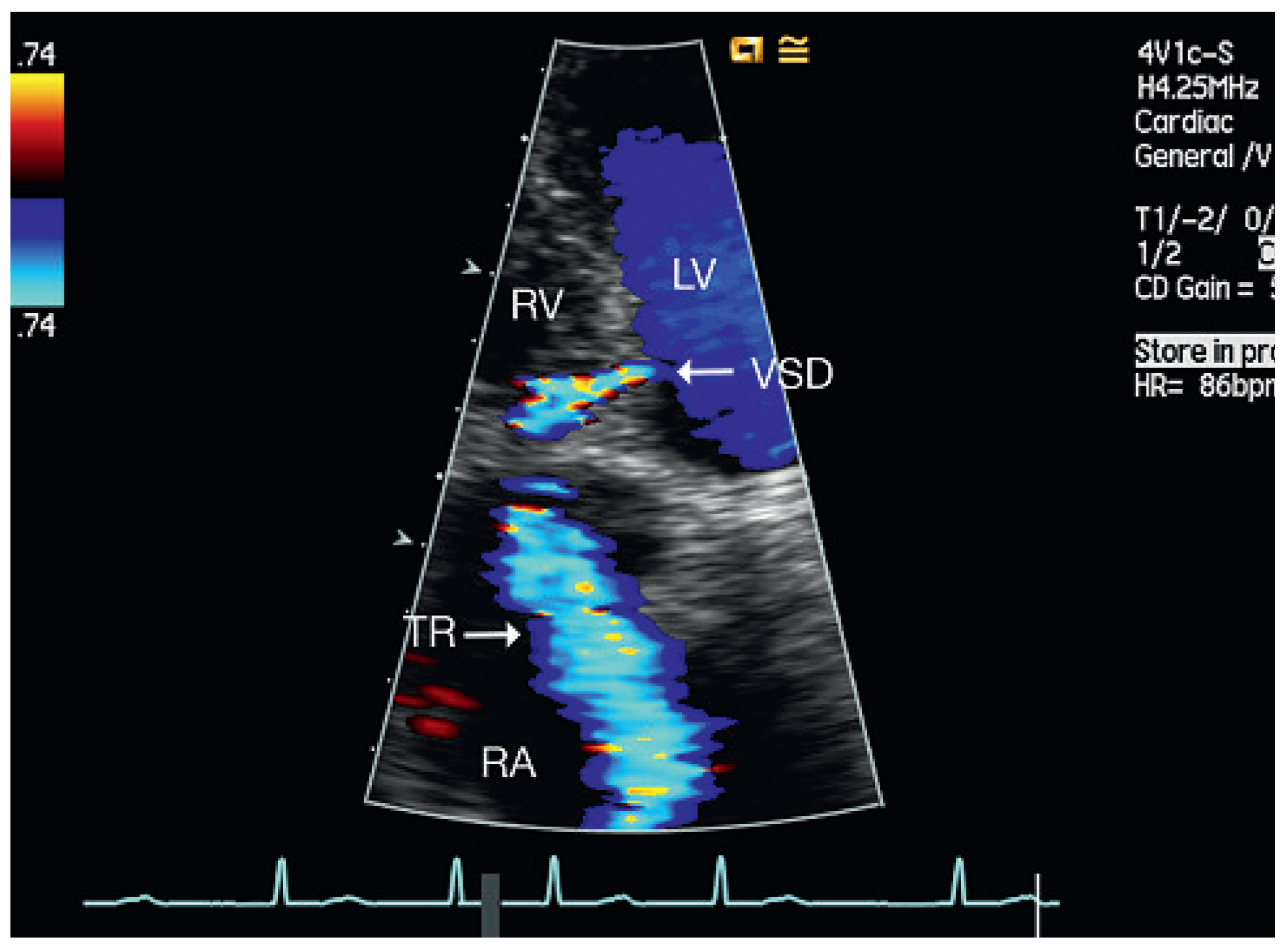
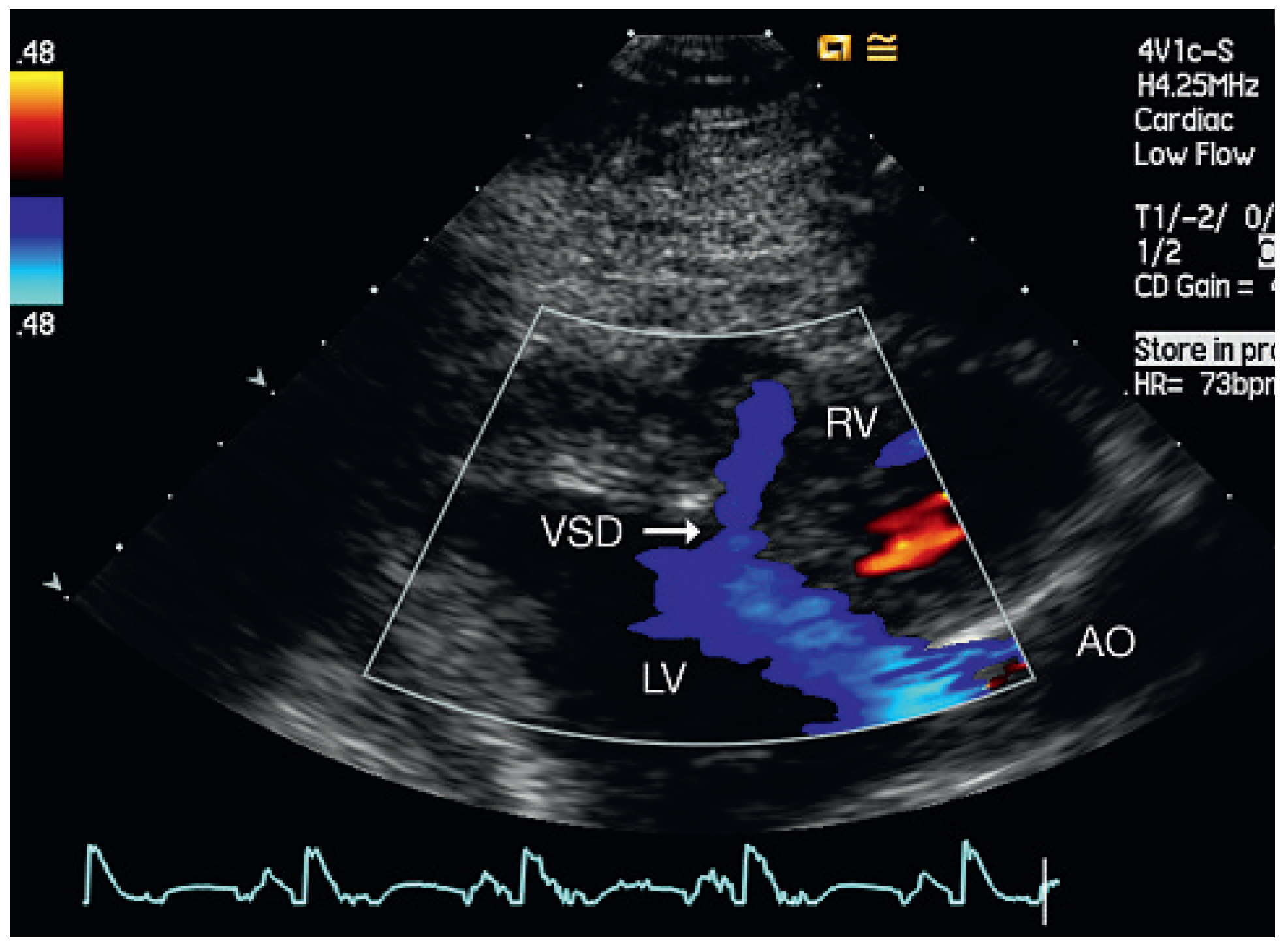
Discussion
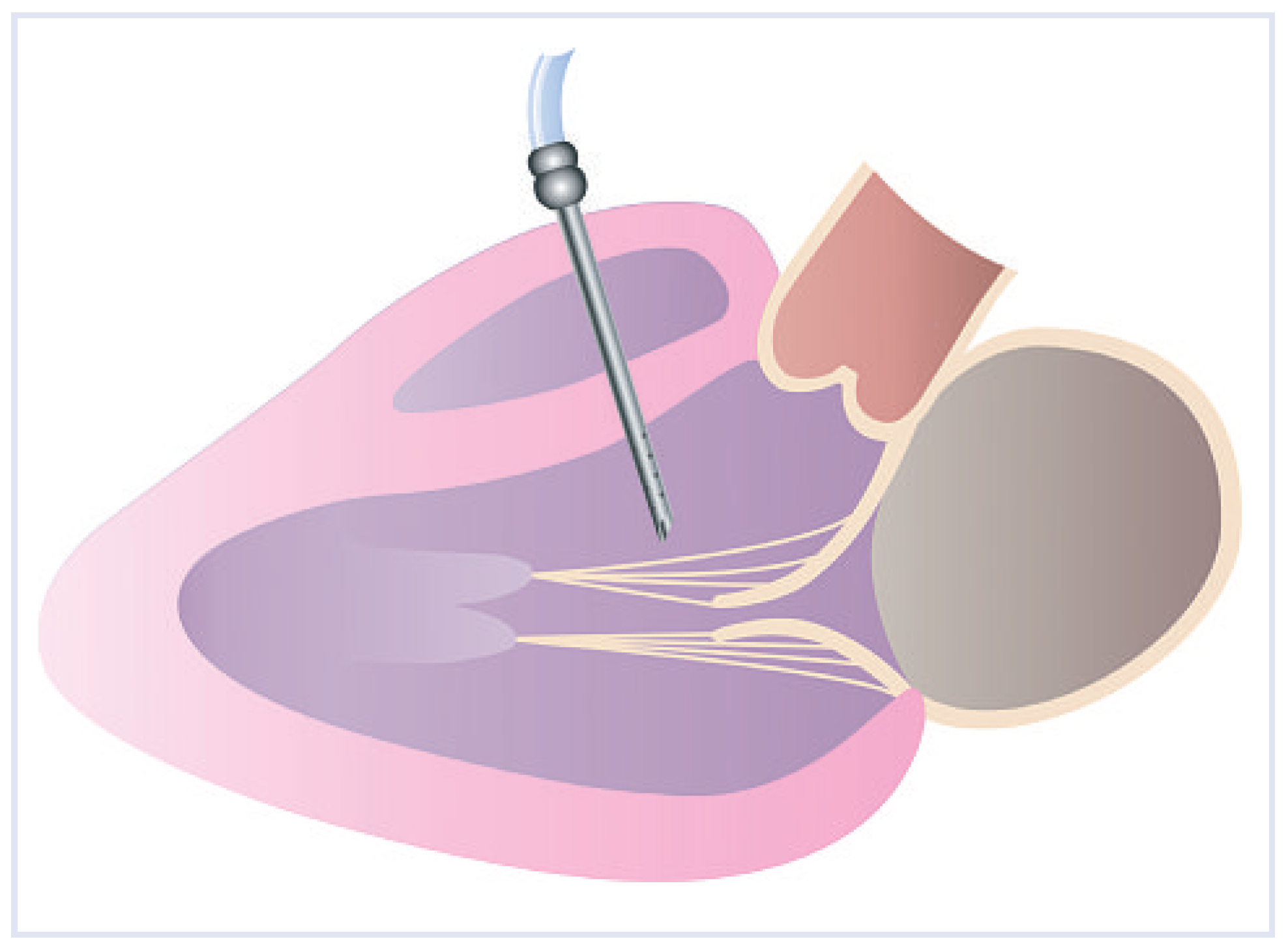

Conclusions
Conflicts of Interest
References
- Weidman WH, DuShane JW, Ellison RC. Clinical course in adults with ventricular septal defect. Circulation 1977, 56 (Suppl. I), 178–179.
- Brickner ME, Hillis LD, Lang RA. Congenital Heart Disease in Adults. N Engl J Med. 2000, 342, 256–262. [CrossRef] [PubMed]
- Crenshaw BS, Granger CB, Birnbaum Y; et al. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation 2000, 101, 27–32.
- Dehghani P, Ibrahim R, Collins N, Latter D, CheemaAN, Chisholm RJ. Post-traumatic ventricular septal defects—Review of the literature and a novel technique for percutaneous closure. J Invasive Cardiol. 2009, 21, 483–487.
- Berry C, Hillis WS, Knight WB. Transcatheter closure of a ventricular septal defect resulting from knife stabbing using the Amplatzer muscular VSD occluder. Catheter Cardiovasc Interv. 2006, 68, 153–156.
- Martinez MW, Mookadam F, Sun Y, Hagler DJ. Transcatheter closure of ischemic and post-traumatic ventricular septal ruptures. Catheter Cardiovasc Interv. 2007, 69, 403–407.
- Villanueva FS, Heinsiner JA, Burkman MH, Fonanapazir L, Halvorsen RA, Jr, Chen JT. Echocardiographic detection of perforation of the cardiac ventricular septum by a permanent pacemaker lead. Am J Cardiol. 1987, 59, 370–371. [CrossRef] [PubMed]
- Park JY, Park SH, Oh JY, Kim IJ, Lee YH, Park SH, Kwon KH. Delayed ventricular septal rupture after percutaneous coronary intervention in acute myocardial infarction. Korean J Intern Med. 2005, 20, 243–246. [CrossRef] [PubMed]
- Nishimura RA, Holmes, Jr. DR. Hypertrophic obstructive cardiomyopathy. N Engl J Med. 350, 1320–1327. [CrossRef] [PubMed]
- Dodos F, Fehske W, Hoppe U, Sreeram N. Thranscatheter closure of iatrogenic perimembranous ventricular septal defect following prosthetic aortic valve replacement. Clin Res Cardiol. 2008, 97, 53–55. [CrossRef] [PubMed]
- Noble S, Ibrahim R. Transcatheter membranous ventricular septal defect closure through a mechanical aortic prosthesis using the Amplatzer membranous ventricular septal defect occluder. Catheter Cardiovasc Interv. 2009, 73, 167–172.
- Klein AJ, Garcia JA, Carrol JD. Percutaneous closure of an iatrogenic ventricular septal defect following mechanical aortic valve replacement using transseptal technique. Catheter Cardiovasc Interv. 2007, 70, 1018–1024.
- Hedlund, K.D. A tribute to Frank F. Allbritten, Jr. Texas Heart Inst J. 2001, 28, 292–296. [Google Scholar]
- Hedlund, K.D. Origin of the left ventricular vent. Texas Heart Inst J. 2004, 31, 107–108. [Google Scholar]
- Groves LK, Effler DB. Needle-Vent safeguard against systemic air embolus in open-heart surgery. J Thorac Cardiovasc Surg. 1964, 47, 349–355. [CrossRef]
- Sa MI, Lli Wie, Sheppard MN, Kilner PJ. Mycotic left ventricular false aneurysm at the site of an apical vent presenting 24 years after aortic valve surgery. Circulation 2008, 118, e501–e503.
- Strassano P, DeAmicis V, Gagliardi C, Di Lello F, Stampinato N. False aneurysm from the aortic vent site. J Cardiovasc Surg (Torino) 1982, 23, 401–402.
- Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M; et al.; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; American Heart Association Council on Cardiovascular Disease in the Young; American Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Surgery andAnesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group. Prevention of infective endocarditis: Guidelines from the American Heart Association: Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; American Heart Association Council on Cardiovascular Disease in the Young; American Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007, 116, 1736–1754, Erratum in: Circulation 2007, 116, e376–e377.
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA; et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008, 118, e714–e833.
| Age | Gender | Diagnosis | Valve | Size of | NYHA Class | VSD | LVDD, | LAD, |
|---|---|---|---|---|---|---|---|---|
| Prosthesis | Prosthesis | at Admission | Location | mm | mm | |||
| 54 | Male | Combined aortic stenosis with regurgitation | ATS | 23 mm | II | Mid-septal | 46 | 40 |
| 59 | Male | Severe aortic regurgitation | ATS | 23 mm | II | Anteroseptal basal | 50 | 46 |
| 59 | Male | Severe mitral regurgitation due to chordal rupture | ATS | 31 mm | I | Mid-basal septum | 48 | 45 |
| 69 | Female | Combined aortic stenosis with regurgitation | ATS | 20 mm | II | Mid-septobasal | 50 | 54 |
| 61 | Male | Severe aortic stenosis | ATS | 22 mm | II | Anteroseptal | 55 | 60 |
© 2010 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Aragão, A.; Vogel, D.; Schmidt, C. Muscular Ventricular Septal Defect After Mitral and Aortic Valve Replacement. Cardiovasc. Med. 2010, 13, 167. https://doi.org/10.4414/cvm.2010.01500
Aragão A, Vogel D, Schmidt C. Muscular Ventricular Septal Defect After Mitral and Aortic Valve Replacement. Cardiovascular Medicine. 2010; 13(5):167. https://doi.org/10.4414/cvm.2010.01500
Chicago/Turabian StyleAragão, Augusto, Dorothea Vogel, and Christoph Schmidt. 2010. "Muscular Ventricular Septal Defect After Mitral and Aortic Valve Replacement" Cardiovascular Medicine 13, no. 5: 167. https://doi.org/10.4414/cvm.2010.01500
APA StyleAragão, A., Vogel, D., & Schmidt, C. (2010). Muscular Ventricular Septal Defect After Mitral and Aortic Valve Replacement. Cardiovascular Medicine, 13(5), 167. https://doi.org/10.4414/cvm.2010.01500



