Abstract
Imatinib mesylate, a selective inhibitor of tyrosine kinases, has excellent efficacy in the treatment of chronic myeloid leukaemia (CML) and gastrointestinal stromal tumour (GIST). Inducing durable responses and achieving prolonged survival, it has become the standard of care for the treatment of these diseases. It has opened the way to the development of additional tyrosine kinase inhibitors (TKIs), including sunitinib, nilotinib, dasatinib and sorafenib, all indicated for the treatment of various haematological malignancies and solid tumours. TKIs are prescribed for prolonged periods and are often taken by patients with—notably cardiovascular—comorbidities. Hence TKIs are regularly co-administered with cardiovascular drugs, with a considerable risk of potentially harmful drug-drug interactions due to the large number of agents used in combination. However, this aspect has received limited attention so far, and a comprehensive review of the published data on this important topic has been lacking. We review here the available data and pharmacological mechanisms of interactions between commonly prescribed cardiovascular drugs and the TKIs marketed at present. Regular updating of the literature on this topic will be mandatory, as will the prospective reporting of unexpected clinical observations, given the fact that these drugs have been only recently marketed.
Introduction
Targeted cancer therapies have been designed to interact with particular proteins associated with tumour development or progression. Many of these agents are tyrosine kinase inhibitors (TKIs), targeting enzymes whose dysregulated expression and activity are associated with various types of cancer [1]. The pioneer small-molecule TKI imatinib has revolutionised the treatment and prognosis of chronic myeloid leukaemia (CML) and gastrointestinal stromal tumour (GIST) [2,3].
Imatinib was designed [4] to inhibit the tyrosine kinase Bcr-Abl [5], a fusion oncoprotein resulting from the translocation t(9;22)(q34;q11), which produces the characteristic Philadelphia chromosome [5], the hallmark of CML and of some acute lymphoblastic leukaemia (ALL) [6].
Imatinib was also found to be a potent inhibitor of two additional tyrosine kinases, namely KIT, involved in the oncogenesis of GIST [7,8,9], and platelet-derived growth factor receptor (PDGFR) involved in the pathogenesis of the hypereosinophilic syndrome [10].
Following imatinib, several other TKIs, including sunitinib, nilotinib, dasatinib and sorafenib, have been developed and are now used in the treatment of various haematological malignancies, solid tumours including GIST, advanced renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC), while showing promising activity in other malignancies as well [11]. TKIs are extensively metabolised by cytochromes P450, whose activity is characterised by a large degree of inter-individual variability [12]. Some of them are also substrate or inhibitors of the drug transporters P-glycoprotein (Pgp = ABCB1), breast cancer resistance protein (BCRP = ABCG2) and the organic cation carrier hOCT1 (SLC22A1). A given dose may therefore yield very different circulating concentration profiles from one patient to another, thus favouring the selection of resistant cellular clones in the event of sub-therapeutic drug exposure or the occurrence of undesirable toxicity in cases of overexposure.
Identifying the most active and safest dosing schedule for individual patients to maximize therapeutic benefit has turned out to be a scientific and clinical challenge. Combination therapies have been investigated in various conditions, which certainly add a level of treatment complexity since overlapping toxicities and pharmacokinetic interactions must be carefully monitored.
The small-molecule TKIs developed to date share a roughly similar safety profile and are generally better tolerated than traditional cytotoxic chemotherapies. They are however administered over prolonged periods, if not indefinitely, and are often taken by patients with comorbidities, notably cardiovascular disorders. Thus, TKIs are likely to be administered simultaneously with other treatments, in particular cardiovascular agents with a potential risk of harmful drug-drug interactions.
Furthermore, there have been reports that TKIs themselves may cause cardiotoxicity on their own [13,14,15]. In some patients at least they cause symptomatic congestive heart failure or asymptomatic left ventricular dysfunction [13,15,16]. TKIs do indeed to some extent inhibit normal variants of tyrosine kinases in noncancerous cells, which could explain such adverse effects. The actual importance of such toxicities remains to be confirmed in additional studies.
Recently confronted with an increasing number of requests concerning drug interactions of TKIs with cardiovascular drugs, we have decided to review systematically the data available on pharmacological interactions between commonly prescribed cardiovascular agents and TKIs. Interactions between TKIs and cytochrome P450 inhibitors prescribed for a limited period of time, such as antibiotics, antifungals [17], or inducers such as rifampicin [18], have been previously described and will not be the principal focus of the present review, which emphasises the potential interactions between TKIs and cardiovascular drugs taken indefinitely by patients presenting cardiovascular comorbidities. Table 1 has been devised as a tool to enable practitioners to improve safety in prescribing such drug combinations. It does however not replace medical evaluation and should be used in addition to thoroughly weighed clinical judgment. Actually, most interactions do not represent true contraindications but rather call for appropriate dosage adjustment and treatment monitoring measures.
Review of the Literature
Initial information was gathered from the official Swiss drug information source “Compendium Suisse des Médicaments 2009” [19]. In a second step, literature from Medline and evidence-based medicine reviews was systematically searched using the following MeSH terms: “drug interactions”, “cytochrome p450 enzyme system”, “p-glycoprotein”, “protein binding”, the respective TKIs names and common cardiovascular drugs. Additionally, two drug information databases (UpToDate online [20] and Cancer Care Ontario [21]) were screened, and abstracts of international and national conferences, review articles and references given in identified articles were also scanned [22,23,24]. All relevant literature on pharmacokinetic or pharmacodynamic interactions was considered for inclusion in Table 1.
Drug interactions were either clinically documented or derived from considerations on proven or putative metabolic pathways, protein binding and transmembrane transport of cardiovascular drugs and TKIs. When data on a particular combination were unavailable, potential interactions were predicted from the reported disposition mechanisms of the agents.
Interaction Between Cardiovascular Drugs and Tyrosine Kinase Inhibitors
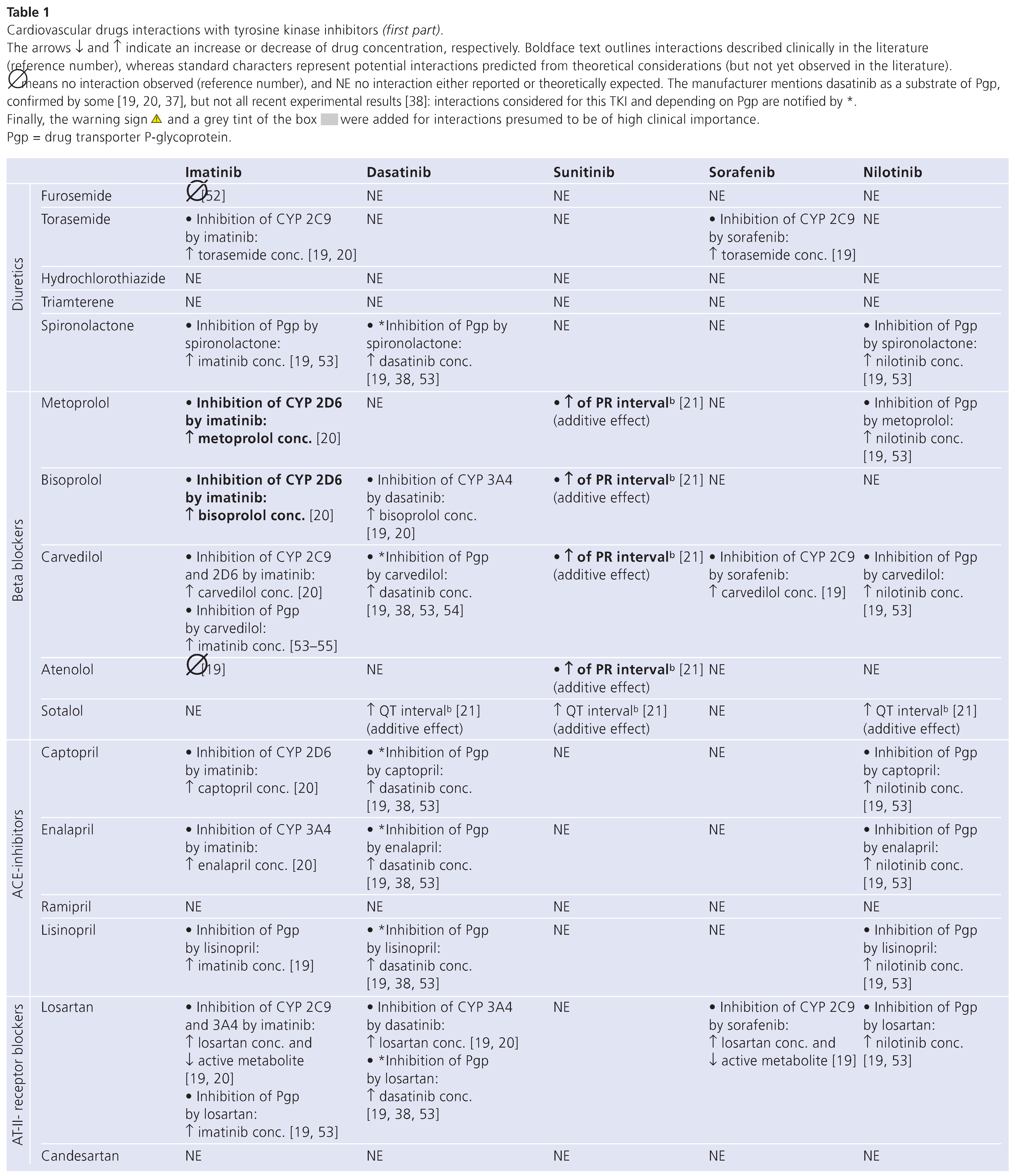
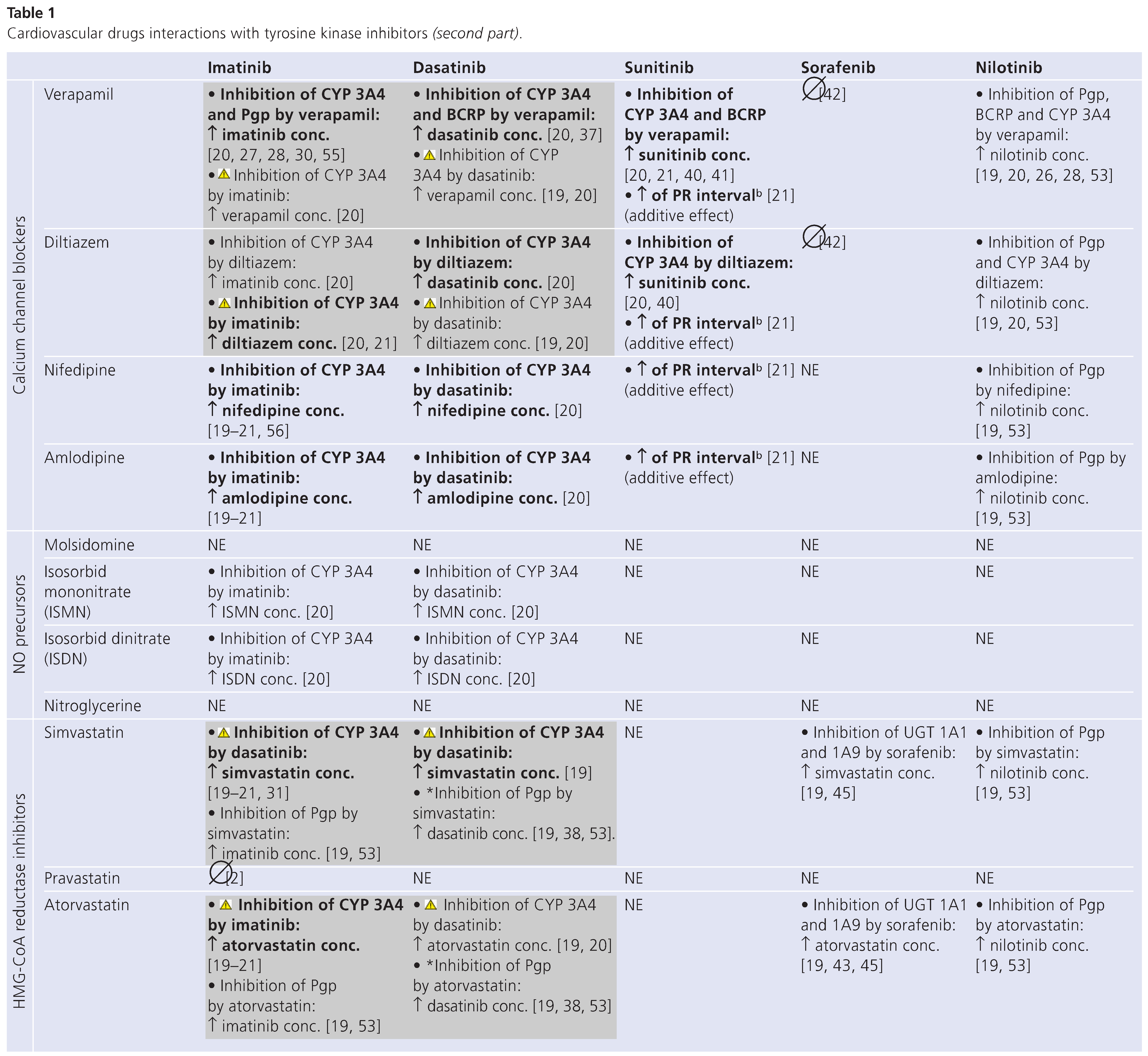
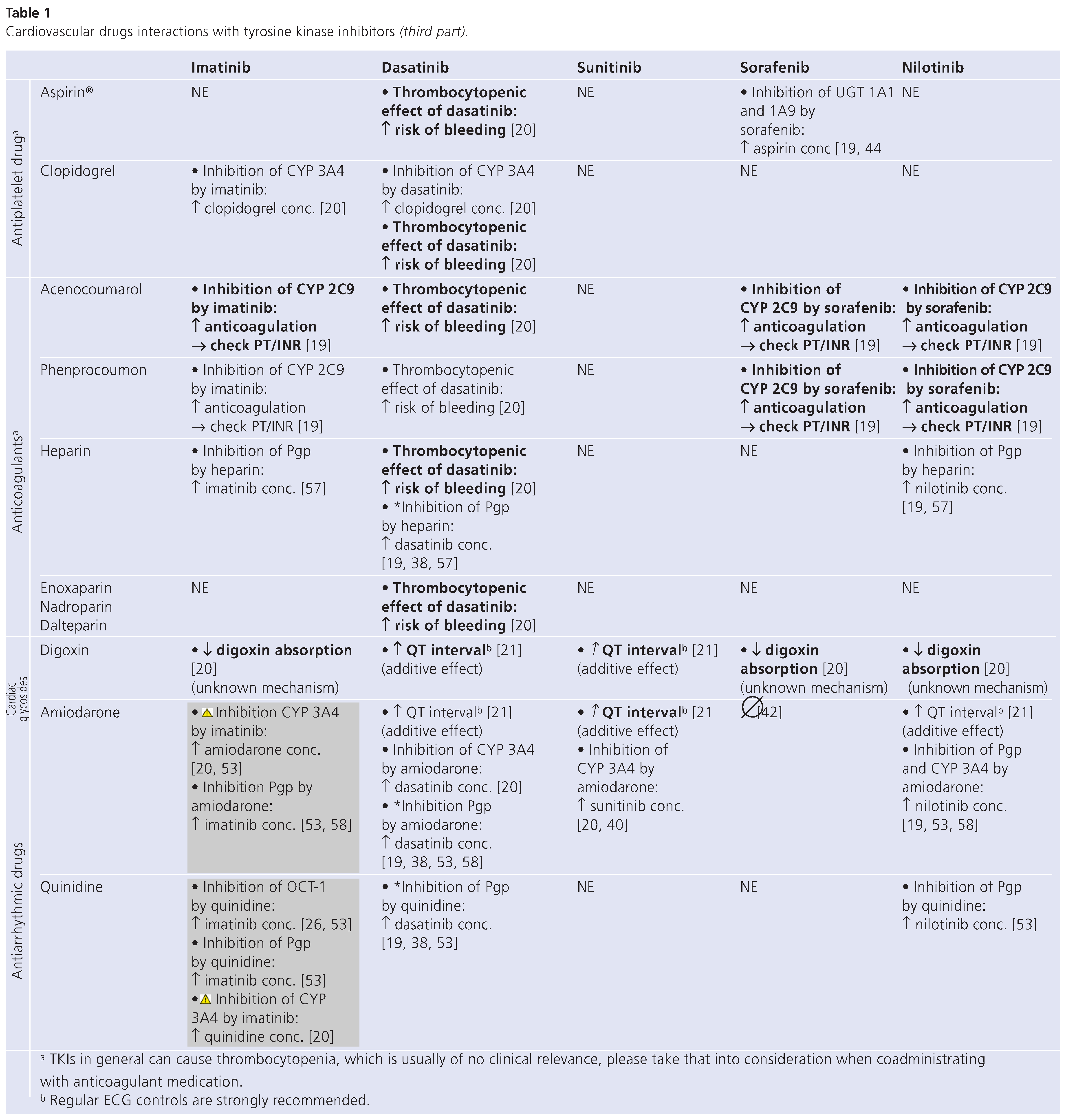
Table 1 (in 3 parts) summarises known or potential drug interactions between commonly prescribed cardiovascular drugs (lines) and the five major TKIs commercially available at present (columns). Each cell indicates the type of interaction expected.
Interactions with Imatinib
Imatinib is metabolised mainly by the cytochrome P450 (CYP) isoenzymes 3A4 and 3A5, while CYP1A2, CYP2D6, CYP2C9 and CYP2C19 play a minor role in its metabolism [25]. This TKI has also been shown to be a substrate of hOCT-1, Pgp and BCRP [19,26,27,28]. However, a controversial report [29] suggests that imatinib is an inhibitor rather than a substrate of BCRP. Hence interactions between imatinib and inhibitors of BCRP [30] are not considered in this article. The metabolites of imatinib are eliminated predominantly through the bile, one metabolite (CGP 74588) showing comparable pharmacological activity to the parent drug, but amounting to less than 20% of circulating imatinib concentration. The faecal to urinary excretion ratio is approximately 5:1 [25]. Interactions may occur between imatinib and inhibitors or inducers of CYP3A4/5 and Pgp, leading to changes not only in the plasma but also in the cell concentrations of imatinib. For example, verapamil, a CYP3A4 and Pgp inhibitor [19], and carvedilol, a Pgp inhibitor, increase intracellular concentrations of imatinib by decreasing its metabolism and inhibiting its efflux via Pgp, and hence may increase the cellular toxicity of imatinib.
Moreover, this TKI may competitively inhibit the metabolism of drugs that are CYP3A4, CYP3A5, CYP2D6 and CYP2C9 substrates. Interactions of potential clinical relevance can thus occur with calcium channel blockers such as verapamil and diltiazem, substrates of CYP3A4, whose circulating levels are increased when associated with imatinib [20,21]. Interactions with simvastatin, atorvastatin, amiodarone and quinidine, involving the same P450 isoenzyme, may also be of clinical relevance [19,20,21,31]. In patients taking imatinib these drugs should be avoided whenever possible and replaced by safer alternatives (e.g., pravastatin or sotalol) [20,32].
Finally, the interaction with quinidine, a known inhibitor of hOCT-1, may paradoxically increase the circulating concentrations of imatinib but decrease the exposure of target cancer cells known to express this carrier [19,26]. With regard to all these mechanisms, it is worth noting that plasma concentrations of imatinib are correlated with efficacy and toxicity [33,34,35,36]. A change in imatinib exposure due to a drug interaction may therefore directly influence its therapeutic effect.
Interactions with Dasatinib
Dasatinib is metabolised to an active metabolite and other inactive metabolites by the CYP3A4 isoenzyme, and was also reported to be a substrate of BCRP and Pgp [19,20,37], though this has recently been questioned in pre-clinical models [38]. The active metabolite does not appear to play a significant role in dasatinib’s therapeutic activity. It has weak inhibitory activity against CYP3A4. Concomitant administration of drugs that inhibit CYP3A4/5 and BCRP, such as verapamil, may lead to an increase in dasatinib exposure, which raises the risk of cumulative cardiac toxicity. Conversely, concomitant administration of CYP3A4/5 inducers may lead to a reduction of as much as 80% in dasatinib exposure [38,39]. In association with cardiovascular drugs, the same relevant interactions as with imatinib have been reported for dasatinib [19,20], and concurrent use of the drugs concerned should also be avoided [20,32].
Interactions with Sunitinib
In vitro studies have determined sunitinib metabolism to be mediated primarily by the CYP3A4 isozyme [40]. Two N-deethylation steps are required to render sunitinib inactive. An active metabolite is formed after the first N-deethylation step mediated by CYP3A4. The active metabolite is further metabolised by CYP3A4, but at a lower rate than in the first step, to form an inactive metabolite (SU14335). An increase of about 50% in total sunitinib exposure has been observed when sunitinib was given concomitantly with ketoconazole, a potent CYP3A4/5 inhibitor [40]. To adjust for this increase, it is recommended in patients receiving strong CYP3A4/5 inhibitors that the sunitinib dose be reduced to 66% of the recommended dose [40]. Similarly, healthy volunteers receiving rifampin, a strong CYP3A4/5 inducer, had a 50% decrease in combined systemic exposure to sunitinib [40]. To adjust for this decrease, it is recommended in patients who require concomitant use of a CYP3A4/5 inducer that the sunitinib dose be increased to 175% of the recommended dose [40]. As shown in Table 1, sunitinib was found to be one of the TKIs affecting the least the disposition of cardiovascular drugs. An increase in PR interval can occur in association with beta-blockers and calcium channel blockers, and an increase in QT interval with digoxine and amiodarone [21]. However, the only pharmacokinetic interactions found were with verapamil, diltiazem and amiodarone [20,21,40,41].
Interactions with Sorafenib
Sorafenib is eliminated by a combination of CYP3A4–mediated oxidative metabolism, phase II glucuronidation, and (possibly) biliary secretion, with glucuronidated metabolites accounting for approximately 19% of an oral dose [42]. During co-administration with ketoconazole, there was no increase in sorafenib exposure values, and no change in terminal elimination half-life, compared to sorafenib alone [42]. The results suggest that sorafenib may be safely administered with drugs known to inhibit CYP3A4/5–mediated metabolism without dosage adjustment. However, sorafenib is an inhibitor of UDP-glucurunosyl transferase (UGT) 1A1, and 1A9, as well as CYP2C9, theoretically leading to an increase in plasma concentrations of CYP2C9 substrates, such as torasemide, carvedilol, losartan, acenocoumnarol and phenprocoumon [19]. Moreover, aspirin, simvastatin and atorvastatin are substrates of UGT1A1 and 1A9, and consequently their concentration may increase when combined with sorafenib [19,43,44,45]. There are no data available describing clinical consequences of such combinations.
Interactions with Nilotinib
The risk of clinically relevant drug interactions with nilotinib is poorly documented. The drug undergoes metabolism by CYP3A4, and concomitant administration of strong inhibitors or inducers of CYP3A4/5 are expected to increase or decrease nilotinib concentrations significantly. In healthy subjects receiving ketoconazole, systemic exposure (AUC) to nilotinib was increased approximately 3–fold [19]. This TKI is also a substrate of the efflux transporters Pgp and BCRP [19,28]. When administered with Pgp and BCRP inhibitors, increased concentrations of nilotinib are therefore expected [19,20,28].
At present, nilotinib is known to inhibit CYP2C9. Acenocoumarol and phenprocoumon, substrates of CYP2C9, show increased concentrations, imposing careful monitoring of PT/INR [19]. A fact of note is that nilotinib, also known to inhibit UGT1A1 [46], has been found to increase bilirubin levels, and the largest increase occurs in patients homozygous for the UGT1A1*28 reduced-function variant.
Discussion
The treatment of cancer patients has shifted from traditional, non-specific cytotoxic chemotherapy cycles to chronic treatment with molecular targeted therapies. Drug interactions with cardiovascular agents simultaneously prescribed may cause potentially harming drug-drug interactions in patients treated with TKIs [42]. Most of the interactions outlined in Table 1 (excepted those in boldface) are theoretical and have not been confirmed in clinical studies; therefore they should only be considered indicative; further interaction mechanisms may still be unknown. Moreover, not all interactions are expected to bear clinical significance and/or to imply dosage adjustment.
Besides pharmacokinetic interactions, TKIs can also cause cardiovascular toxicities on their own, which may further complicate therapeutic management. Patients with cardiovascular disease, particularly those with impaired left ventricular function, should be closely monitored when starting a TKI. As demonstrated by Chu et al. [13], the initiation of sunitinib was associated with heart failure and worsening systolic function in a significant number of patients with underlying cardiovascular disease. Similarly, Kerkela et al. [15] suggest that imatinib is cardiotoxic and can lead to severe left ventricular dysfunction and heart failure. However, there is ongoing controversy about potential cardiotoxic effects of imatinib [47].
Moreover, anti-VEGF agents, as sunitinib and sorafenib, can cause multiple manifestations of endothelial damage, with hypertension and thrombotic microangiopathy [48,49]. Physicians should be aware of these potential associations, as early recognition and prompt therapeutic intervention can be beneficial.
In clinical trials, nilotinib treatment has been associated with prolongation of the QTc interval, and cases of sudden cardiac death have occurred, probably related to ventricular repolarisation abnormalities [50]. The prescribing information for nilotinib carries a black box warning regarding the risk of these events.
Dasatinib is also known to cause cardiac disorders, such as QT prolongation, oedema, pleural/pericardial effusion, bleeding, compromised left ventricular function and congestive heart failure [14,16].
Pharmacokinetic drug interactions and cardiovascular safety are best characterised for imatinib, which was the first TKI on the market. The other TKIs, just recently marketed, have so far only a limited documentation of clinically relevant interactions. This article is up to date as of May 2009, but we advise the reader to regularly check for updates regarding this subject. Documenting unexpected observations and reporting them to the Pharmacovigilance network is therefore necessary. Finally, a Therapeutic Drug Monitoring Service is available for TKIs at the Division of Clinical Pharmacology at CHUV [51] and should be considered when physicians are looking for information on TKIs plasma drug exposure in their patients, when a drug interaction is suspected, or in the event of toxicity, or lack of expected clinical response.
Financial support/Conflict of Interest
Educational Grant from Novartis Pharma Schweiz AG.
References
- Krause DS, Van Etten RA. Tyrosine Kinases as Targets for Cancer Therapy. N Engl J Med. 2005, 353, 172–187. [CrossRef]
- Apperley, J.F. Part I: Mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007, 8, 1018–1029. [Google Scholar] [CrossRef]
- Badalamenti G, Rodolico V, Fulfaro F, Cascio S, Cipolla C, Cicero G; et al. Gastrointestinal stromal tumours (GISTs): Focus on histopathological diagnosis and biomolecular features. Ann Oncol. 2007, 18 (Suppl. 6), vi136–vi140. [CrossRef]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S; et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996, 2, 561–566. [CrossRef] [PubMed]
- Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 1990, 247, 1079–1082. [CrossRef] [PubMed]
- Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002, 1, 493–502. [CrossRef]
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ; et al. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumours. N Engl J Med. 2002, 347, 472–480. [CrossRef] [PubMed]
- Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 2000, 96, 925–932. [CrossRef]
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S; et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumours. Science 1998, 279, 577–580. [CrossRef]
- Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J; et al. A Tyrosine Kinase Created by Fusion of the PDGFRA and FIP1L1 Genes as a Therapeutic Target of Imatinib in Idiopathic Hypereosinophilic Syndrome. N Engl J Med. 2003, 348, 1201–1214. [CrossRef]
- Le Tourneau C, Faivre S, Raymond E. New developments in multitargeted therapy for patients with solid tumours. Cancer Treat Rev. 2008, 34, 37–48. [CrossRef]
- Rochat B, Fayet A, Widmer N, Lahrichi SL, Pesse B, Decosterd LA, Biollaz J. Imatinib metabolite profiling in parallel to imatinib quantification in plasma of treated patients using liquid chromatography-mass spectrometry. J Mass Spectrom. 2008, 43, 736–752. [CrossRef]
- Chu, T.F. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007, 370, 2011–2019. [Google Scholar] [CrossRef]
- Force T, Kerkela R. Cardiotoxicity of the new cancer therapeutics—Mechanisms of, and approaches to, the problem. Drug Discov Today. 2008, 13, 778–784. [CrossRef]
- Kerkela, R. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006, 12, 908–916. [Google Scholar] [CrossRef]
- Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007, 7, 332–344. [CrossRef]
- Dutreix C, Peng B, Mehring G, Hayes M, Capdeville R, Pokorny R, Seiberling M. Pharmacokinetic interaction between ketoconazole and imatinib mesylate (Glivec) in healthy subjects. Cancer Chemother Pharmacol. 2004, 54, 290–294.
- Bolton AE, Peng B, Hubert M, Krebs-Brown A, Capdeville R, Keller U, Seiberling M. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol. 2004, 53, 102–106. [CrossRef] [PubMed]
- Kompendium.ch [homepage on the Internet]. Basel: Compendium Suisse des médicaments 2009 [updated 2009; cited 2009]. Available online: http://www.kompendium.ch/.
- UpToDate.com 2009 [homepage on the Internet]. Waltham: UpToDate 2009 [updated 2009; cited 2009]. Available online: http://www.uptodate. com/.
- Cancercare.on.ca [homepage on the Internet]. Toronto: Cancer Care Ontario. 2009 [updated 2009; cited 2009]. Available online: http://www. cancercare.on.ca/.
- Asco.org [homepage on the Internet]. Alexandria: American Society of Clinical Oncology. [updated 2009; cited 2009]. Available online: http://www.asco.org.
- Clinical care option for Oncology. Clinicalcareoptions.com 2009 [updated 2009; cited 2009]. Available online: http://www.clinicalcareoptions.com/Oncology.aspx.
- Medscape. Medscape.com 2009 [cited 2009]. Available online: http://www. medscape.com.
- Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005, 44, 879–894. [CrossRef] [PubMed]
- White, D.L. OCT-1–mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): Reduced OCT1 activity is the cause of low in vitro sensitivity to imatinib. 2006 Jul 15. Blood 2006, 108, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Ozvegy-Laczka C, Hegedus T, Varady G, Ujhelly O, Schuetz JD, Varadi A, Keri G, Orfi L, Nemet K, Sarkadi B. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004, 65, 1485–1495. [CrossRef] [PubMed]
- Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S; et al. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia 2007, 21, 1267–1275. [CrossRef]
- Junia, V. Melo. Imatinib and ABCG2: Who controls whom? Blood 2006, 108, 1116–1117. [Google Scholar]
- Yamamoto K, Suzu S, Yoshidomi Y, Hiyoshi M, Harada H, Okada S. Erythroblasts highly express the ABC transporter Bcrp1/ABCG2 but do not show the side population (SP) phenotype. Immunol Lett. 2007, 114, 52–58. [CrossRef]
- O’Brien SG, Meinhardt P, Bond E, Beck J, Peng B, Dutreix C; et al. Effects of imatinib mesylate (STI571, Glivec) on the pharmacokinetics of simvastatin, a cytochrome P450 3A4 substrate, in patients with chronic myeloid leukaemia. Br J Cancer. 2003, 89, 1855–1859. [CrossRef]
- Martin, A. Rizack. The Medical Letter Handbook of Adverse Drug Interactions; The Medical Letter, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD; et al. Imatinib Plasma Levels Are Correlated With Clinical Benefit in Patients With Unresectable/Metastatic Gastrointestinal Stromal Tumours. J Clin Oncol. 2009, 27, 3141–3147. [CrossRef]
- Larson RA, Druker BJ, Guilhot F, O’Brien SG, Riviere GJ, Krahnke T; et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: A subanalysis of the IRIS study. Blood 2008, 111, 4022–4028. [CrossRef]
- Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA; et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 2007, 109, 3496–3499. [CrossRef]
- Widmer N, Decosterd LA, Leyvraz S, Duchosal MA, Rosselet A, DebiecRychter M; et al. Relationship of imatinib-free plasma levels and target genotype with efficacy and tolerability. Br J Cancer. 2008, 98, 1633–1640. [CrossRef]
- Lagas JS, van Waterschoot RA, van Tilburg VA, Hillebrand MJ, Lankheet N, Rosing H; et al. H. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res. 2009, 15, 2344–2351. [CrossRef] [PubMed]
- Kamath AV, Wang J, Lee FY, Marathe PH. Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): A potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemother Pharmacol. 2008, 61, 365–376. [CrossRef] [PubMed]
- Steinberg, M. Dasatinib: A tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007, 29, 2289–2308. [Google Scholar] [CrossRef] [PubMed]
- Adams VR, Leggas M. Sunitinib Malate for the Treatment of Metastatic Renal Cell Carcinoma and Gastrointestinal Stromal Tumours. Clin Ther. 2007, 29, 1338–1353. [CrossRef]
- Shukla S, Robey RW, Bates SE, Ambudkar SV. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos. 2009, 37, 359–365. [CrossRef] [PubMed]
- Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol. 2006, 57, 685–692. [CrossRef]
- Goosen TC, Bauman JN, Davis JA, Yu C, Hurst SI, Williams JA, Loi CM. Atorvastatin glucuronidation is minimally and nonselectively inhibited by the fibrates gemfibrozil, fenofibrate, and fenofibric acid. Drug Metab Dispos. 2007, 35, 1315–1324. [CrossRef]
- Kuehl GE, Bigler J, Potter JD, Lampe JW. Glucuronidation of the aspirin metabolite salicylic acid by expressed UDP-glucuronosyltransferases and human liver microsomes. Drug Metab Dispos. 2006, 34, 199–202. [CrossRef]
- Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH; et al. Glucuronidation of statins in animals and humans: A novel mechanism of statin lactonization. Drug Metab Dispos. 2002, 30, 505–512. [CrossRef]
- Singer JB, Shou Y, Giles F, Kantarjian HM, Hsu Y, Robeva AS; et al. UGT1A1 promoter polymorphism increases risk of nilotinib-induced hyperbilirubinemia. Leukemia. 2007, 21, 2311–2315. [CrossRef]
- Gambacorti-Passerini C, Tornaghi L, Franceschino A, Piazza R, Corneo G, Pogliani E. In reply to “Cardiotoxicity of the cancer therapeutic agent imatinib mesylate”. Nat Med. 2007, 13, 13–14. [CrossRef]
- Kapiteijn E, Brand A, Kroep J, Gelderblom H. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann Oncol. 2007, 18, 1745–1747. [CrossRef]
- Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 2008, 9, 117–123. [CrossRef]
- Cang S, Liu D. P-loop mutations and novel therapeutic approaches for imatinib failures in chronic myeloid leukemia. J Hematol Oncol. 2008, 1, 15. [CrossRef]
- Haouala A, Zanolari B, Rochat B, Montemurro M, Zaman K, Duchosal MA; et al. Therapeutic Drug Monitoring of the new targeted anticancer agents imatinib, nilotinib, dasatinib, sunitinib, sorafenib and lapatinib by LC tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009, 377, 1982–1996.
- Kanda T, Ohashi M, Makino S, Kaneko K, Matsuki A, Nakagawa S, Hatakeyama K. A successful case of oral molecularly targeted therapy with imatinib for peritoneal metastasis of a gastrointestinal stromal tumour. Int J Clin Oncol. 2003, 8, 180–183. [CrossRef] [PubMed]
- Buclin T, Biollaz J, Diézi J. Transports rénaux de médicaments: Mécanismes et potentiel d’interactions. Med & Hyg. 2004, 62, 682–692.
- Bachmakov I, Werner U, Endress B, Auge D, Fromm MF. Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol. 2006, 20, 273–282. [CrossRef] [PubMed]
- Kakumoto M, Sakaeda T, Takara K, Nakamura T, Kita T, Yagami T; et al. Effects of carvedilol on MDR1-mediated multidrug resistance: Comparison with verapamil. Cancer Sci. 2003, 94, 81–86. [CrossRef]
- Breccia M, D’Andrea M, Alimena G. Can nifedipine and estrogen interaction with imatinib be responsible for gallbladder stone development? Eur J Haematol. 2005, 75, 89–90. [CrossRef]
- Angelini A, Di FC, Ciofani G, Di NM, Baccante G, Di IC; et al. Inhibition of P-glycoprotein-mediated multidrug resistance by unfractionated heparin: A new potential chemosensitizer for cancer therapy. Cancer Biol Ther. 2005, 4, 313–317. [CrossRef]
- Kakumoto M, Takara K, Sakaeda T, Tanigawara Y, Kita T, Okumura K. MDR1-mediated interaction of digoxin with antiarrhythmic or antianginal drugs. Biol Pharm Bull. 2002, 25, 1604–1607. [CrossRef] [PubMed][Green Version]
© 2010 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.