Prevalence of Thrombophilia in Patients Undergoing Closure of Patent Foramen Ovale
Abstract
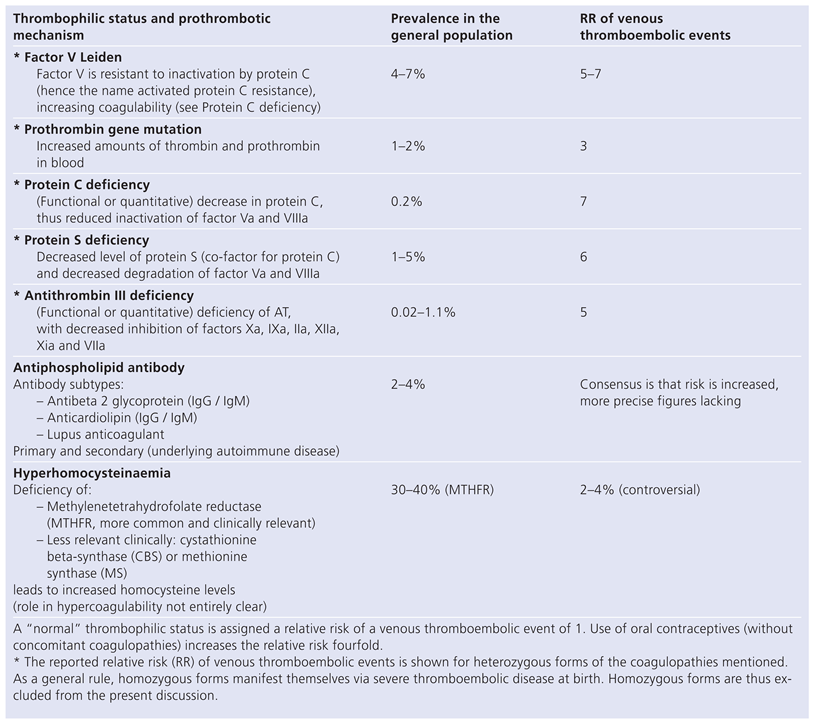
Introduction
Methods
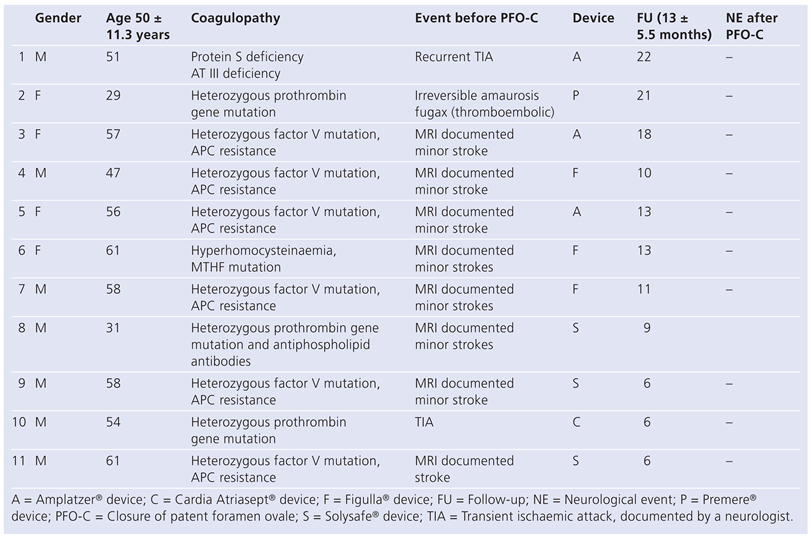
Results
Discussion
Conflicts of Interest
References
- Khairy, P.; O’Donnell, C.P.; Landzberg, M.J. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical emboli: A systematic review. Ann Intern Med. 2003, 139, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Schwerzmann, M.; Windecker, S.; Wahl, A.; Mehta, H.; Nedeltchev, K.; Mattle, H.; et al. Percutaneous closure of patent foramen ovale: Impact of device design on safety and efficacy. Heart 2004, 90, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Kunz, M.; Moschovitis, A.; Nageh, T.; Schwerzmann, M.; Seiler, C.; et al. Long-term results after fluoroscopy-guided closure of patent foramen ovale for secondary prevention of paradoxical embolism. Heart 2008, 94, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Giardini, A.; Donti, A.; Formigari, R.; Bronzetti, G.; Prandstraller, D.; Bonvicini, M.; et al. Comparison of results of percutaneous closure of patent foramen ovale for paradoxical embolism in patients with versus without thrombophilia. Am J Cardiol. 2004, 94, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Krumsdorf, U.; Ostermayer, S.; Billinger, K.; Trepels, T.; Zaden, E.; Horvath, K.; Sievert, H. Incidence and clinical course of thrombus formation on atrial septal defect and patent foramen ovale closure devices in 1000 consecutive patients. J Am Coll Cardiol. 2004, 93, 426–431. [Google Scholar]
- Ruge, H.; Wildhirt, S.M.; Libera, P.; Vogt, M.; Holper, K.; Lange, R. Left atrial thrombus on atrial septal defect closure device as a source of cerebral emboli 3 years after implantation. Circulation 2005, 112, e130–e131. [Google Scholar] [CrossRef] [PubMed]
- Hansson, P.O.; Eriksson, E.; Welin, L.; Eriksson, H. Prevalence of APC resistance and its relationship to arterial and venous thromboembolism in a general population sample of elderly Swedish men. J Internal Med. 1999, 245, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.S.; Miletich, J.P.; Goldhaber, S.Z.; Hennekens, C.H.; Ridker, P.M. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001, 37, 215–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greaves, M.; Cohen, H.; Machin, S.J.; Mackie, I. Guidelines on the investigation and management of the antiphospholipid syndrome. Br J Haematol. 2000, 109, 704–715. [Google Scholar] [CrossRef] [PubMed]
- University of Illinois, Carle Cancer Center, Hematology Resource Page. Available online: www.med.uiuc.edu/hematology.
- Wagdi, P.; Ritter, M. Patient radiation dose during percutaneous interventional closure of interatrial communications. J Cardiol. 2009, 53, 368–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carod Artal, F.J.; Vilela Nunes, S.; Portugal, D. Thrombophilia and patent foramen ovale in young stroke patients. Neurologia 2006, 21, 710–716. [Google Scholar] [PubMed][Green Version]
- Sastry, S.; Riding, G.; Tabemer, D.; Cherry, N.; Heagerty, A.; McCollum, C. Young adult myocardial infarction and ischemic stroke. J Am Coll Cardiol. 2008, 48, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Karttunen, V.; Hiltunen, L.; Rasi, V.; Vahtera, E.; Hilbom, M.; Factor, V. Leiden and prothrombin gene mutation may predispose to paradoxical embolism in subjects with patent foramen ovale. Blood Coagul Fibrinolysis 2003, 14, 261–268. [Google Scholar] [CrossRef] [PubMed]
 |
 |
© 2010 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Wagdi, P.; Ritter, M.; Salzer, F. Prevalence of Thrombophilia in Patients Undergoing Closure of Patent Foramen Ovale. Cardiovasc. Med. 2010, 13, 111. https://doi.org/10.4414/cvm.2010.01494
Wagdi P, Ritter M, Salzer F. Prevalence of Thrombophilia in Patients Undergoing Closure of Patent Foramen Ovale. Cardiovascular Medicine. 2010; 13(4):111. https://doi.org/10.4414/cvm.2010.01494
Chicago/Turabian StyleWagdi, Philipp, Manfred Ritter, and Frank Salzer. 2010. "Prevalence of Thrombophilia in Patients Undergoing Closure of Patent Foramen Ovale" Cardiovascular Medicine 13, no. 4: 111. https://doi.org/10.4414/cvm.2010.01494
APA StyleWagdi, P., Ritter, M., & Salzer, F. (2010). Prevalence of Thrombophilia in Patients Undergoing Closure of Patent Foramen Ovale. Cardiovascular Medicine, 13(4), 111. https://doi.org/10.4414/cvm.2010.01494




