Abstract
Spinal cord stimulation (SCS) is a valuable, last chance treatment for non-reconstructable chronic critical ischemia of the heart or of the limbs. Principally, it induces vasodilation of the microcirculation. Lack of information on the possibilities of SCS is, in part, responsible for non-referral of eligible patients. Whenever feasible, however, surgical or endovascular treatment still remain the best option for treating chronic critical ischemia of the limbs and refractory angina pectoris. Hence, SCS does not represent an alternative to reconstructive procedures, and comparisons with invasive techniques are not pertinent. Systematic macroand microcirculatory tests are essential to identify potential responders. Accordingly, SCS must definitely be regarded as a true vascular treatment requiring the competence of skilled vascular specialists. SCS is a simple, moderately invasive and fully reversible technique, which does not require any incision in ischemic tissues with poor healing capacity. Nowadays, around 75% of carefully, selected patients with refractory angina pectoris or chronic critical limb ischemia obtain substantial and prolonged benefits from SCS treatment. Controlled studies have clearly established that these results are superior to those of the best available conservative treatments. In contrast, however, SCS has no proven benefit in non critical ischemia. The value of new strategies, such as the application of SCS to protect high-risk distal arterial bypasses or ischemic surgical skin flap, is presently under investigation. Along with growing experience, the constant improvement in the selection procedure could lead to repositioning SCS earlier in the treatment algorithm of selected peripheral arterial occlusive disease and angina pectoris patients.
Introduction
Spinal cord stimulation (SCS) consists of the delivery of an intermittent electrical current of limited intensity to the posterior aspect of the spinal cord. SCS has been first introduced into clinical practice by neurosurgeons and pain specialists to treat pain refractory to conventional therapies. Its pain alleviating effect was soon unequivocally demonstrated in patients suffering from neuropathic pain. Basically, SCS is a spin-off, of the gate control theory of pain of Melzack and Wall [1]. The concept of stimulating the dorsal columns of the spinal cord to interfere with the pain processing system is based on multiple and solid anatomical, neurophysiological and clinical data.
The use of SCS to treat peripheral arterial occlusive disease (PAOD) started in 1976, with a report by Cook et al. [2] reporting substantial relief of vascular pain in 9 patients. Eleven years later, Murphy and Giles [3] published similar results in 10 patients suffering from intractable angina pectoris (AP). Within a few years SCS became widely used all over the world. However, the spectacular successes initially reported in PAOD and AP, along with the moderately invasive, non-destructive and fully reversible character of SCS treatment, led to indiscriminate clinical applications and, accordingly, to poor clinical results, and, finally, to prompt disgrace of the method. On the other hand, the observation of the anti-anginal effect of high thoracic SCS gave rise to prolonged controversies focusing on the danger of depriving the patients from the most important warning signal of an imminent and possibly fatal heart attack. The few investigators who decided to continue with SCS began to systematically investigate its mechanisms of action and to look for accurate selection criteria.
The questions to be looked at in this review are the followings:
- What have been the results in recent times?
- What are the putative treatment mechanisms involved in SCS?
- What are the best selection criteria in PAOD and AP patients?
- What are the drawbacks or complications of SCS in PAOD and AP?
- What are the future developments?
Implantation techniques
Patients must be awake and able to cooperate during the lead implantation. Under local anesthesia one or several multipolar (4,8 or 16 poles) leads are inserted into the epidural space either through surgical minilaminectomy or percutaneously. In the simplest and, nowadays, most frequently used percutaneous technique, the electrode is introduced through a Tuohy needle in the epidural space and positioned under fluoroscopic control on the posterior aspect of the spinal cord, around midline, at the high thoracic level for AP and upper limbs stimulation, or at the low thoracic level for lower limb stimulation. Stimulation is begun and the electrode is manipulated until the patient reports comfortable paresthesias covering the whole aching area. The electrode is then anchored to the thoracolumbal fascia and connected to an extension cable linked either to an internal impulse generator immediately implanted in a subcutaneous abdominal pocket, or to a portable external generator for several days of trial stimulation before definitive implantation. The usefulness of a trial stimulation period is still debated. Most authors recommend a trial period of 5 to 10 days through an externalised lead wire. In the author’s experience, trial stimulation is mostly useful to detect patients who are not able to cope with the stimulator. Trial stimulation is also recommended for the patients who hardly fulfil the inclusion criteria for the different vascular indications.
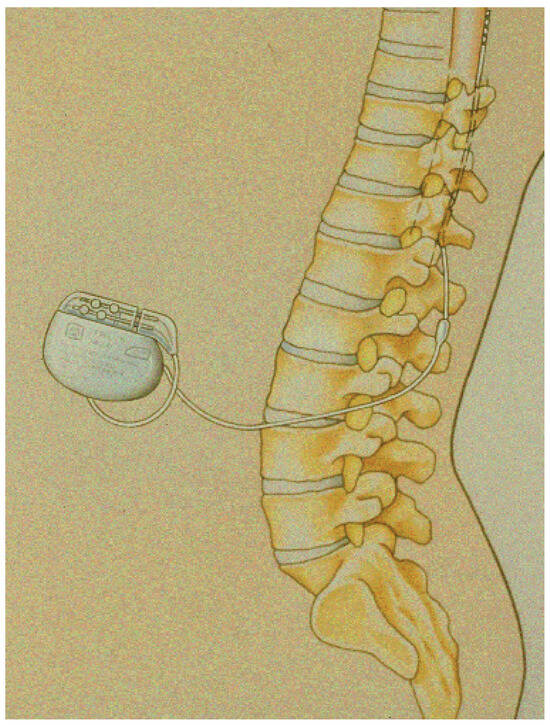
Figure 1.
Schematic representation of a device in place: the tip of the lead has been placed at the low thoracic level and the battery in the subcutaneous tissue of the abdomen.
Figure 1.
Schematic representation of a device in place: the tip of the lead has been placed at the low thoracic level and the battery in the subcutaneous tissue of the abdomen.
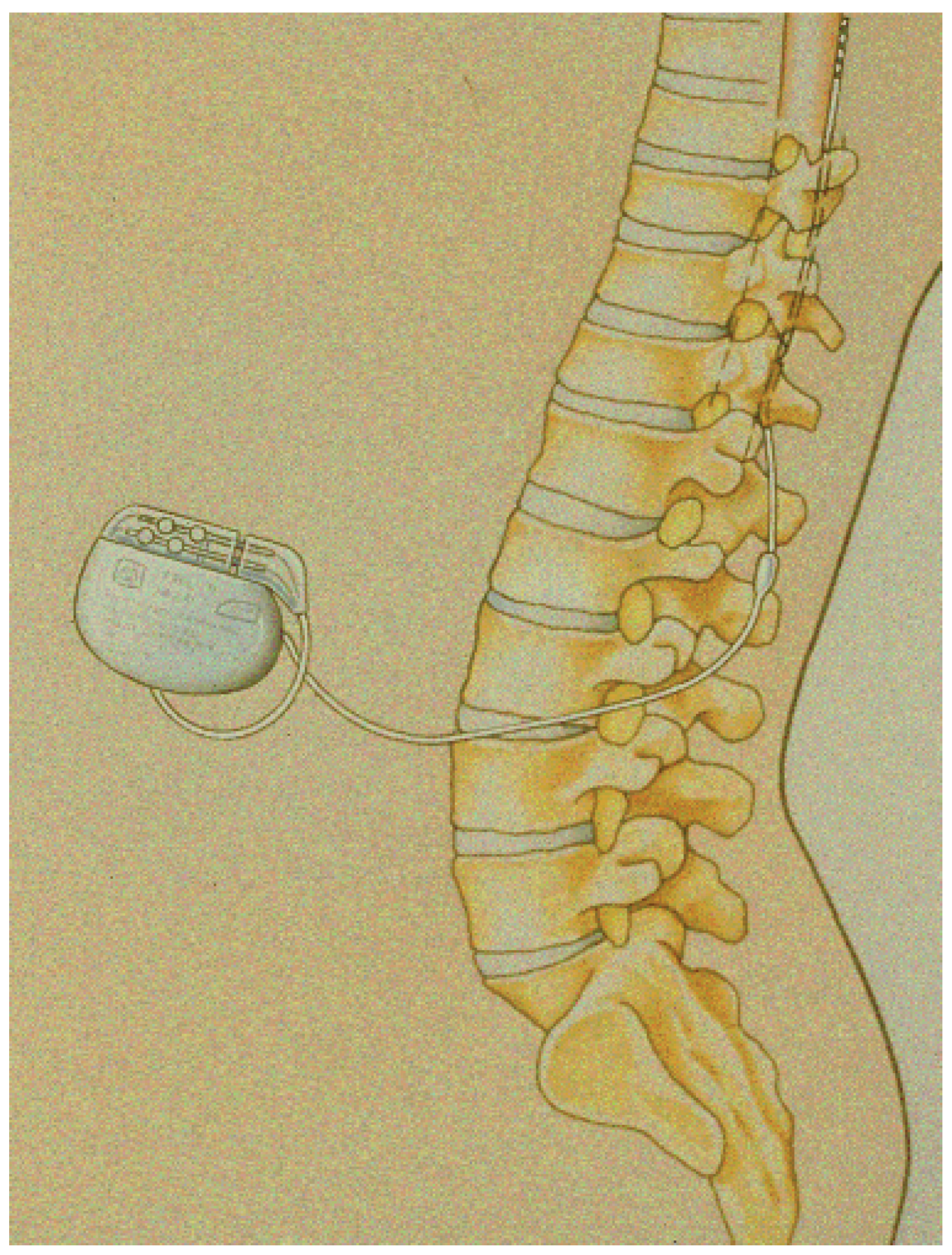
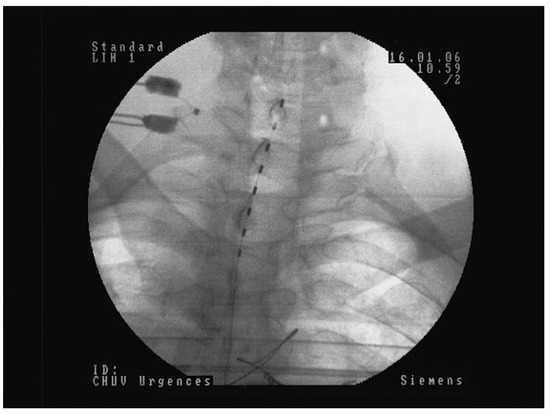
Figure 2.
Octopolar electrode implanted for angina pectoris midline at the thoraco-cervical junction.
Figure 2.
Octopolar electrode implanted for angina pectoris midline at the thoraco-cervical junction.
According to the position of the lead, the number of poles activated and the sensitivity of the patient, the following stimulation parameters are chiefly used: Intensity between 0.1 and 10 volts, pulse width from 50 to 750 μsec and pulse rate 5–120 Hz. Depending on the clinical conditions and vascular indications, various models of implantable generators are available (with single or multiple channels, rechargeable or not etc.). External programming units are available for every model, allowing the resetting, whenever necessary, of all parameters of stimulation. A simpler external device is given to the patient, allowing them to stop and restart stimulation and modify intensity within preset limits.
The choice of the dorsal columns for electrode placement is based on theoretical and practical considerations: the dorsal columns are located in a superficial and easily accessible part of the spinal cord. They are mostly constituted of non-noxious low-threshold large afferents from the limbs and trunk. Electrical activity due to the stimulation of the dorsal columns is distributed orthodromically, as well as antidromically. Holsheimer et al. [4,5] have provided three-dimensional computer studies allowing better understanding of the geometry of the electrical fields created by SCS. Further investigations by the same group have examined the influence of local spinal factors (such as size of the spinal cord and thickness of the cerebrospinal fluid layer at various spinal levels) on the recruitment of the dorsal columns by SCS. This set of data has helped to determine which position of the lead and which pole configuration are most likely to induce optimal stimulation in various clinical conditions.
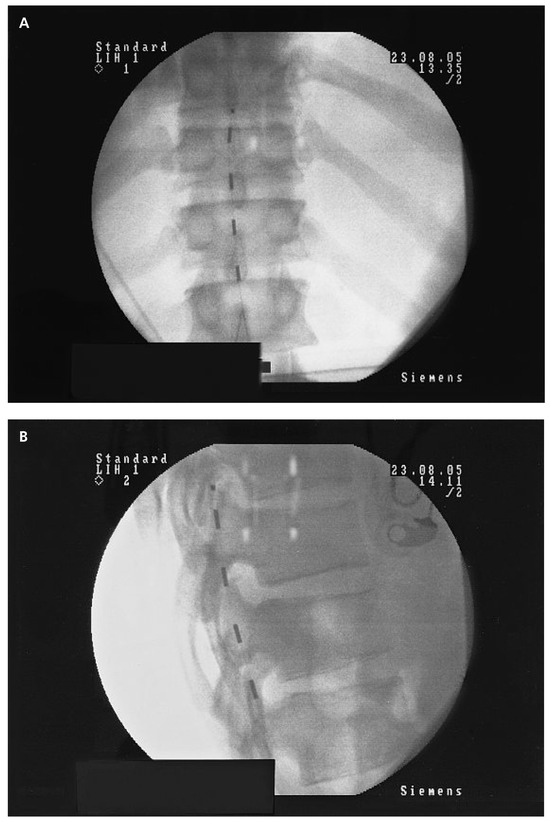
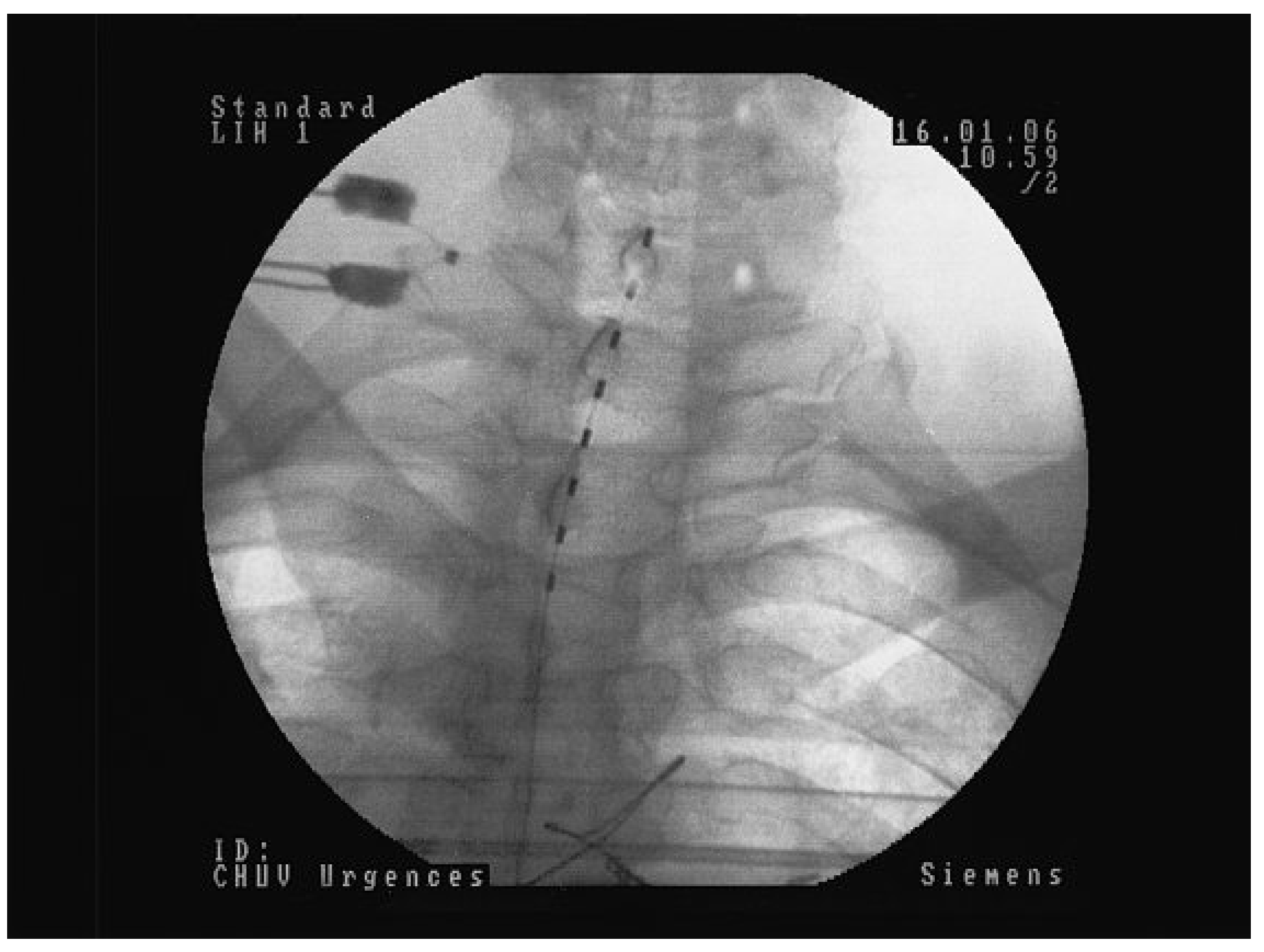
Figure 3.
Quadripolar electrode implanted at the low dorsal level (D11/D12) for PAOD stage IV left and III right: A Postero-anterior view. Note the slight left paramedian position of the electrode in order to increase the effect on the left lower limb. B Lateral view showing the position of the electrode at the posterior part of the spinal canal directly over to the dorsal column.
Figure 3.
Quadripolar electrode implanted at the low dorsal level (D11/D12) for PAOD stage IV left and III right: A Postero-anterior view. Note the slight left paramedian position of the electrode in order to increase the effect on the left lower limb. B Lateral view showing the position of the electrode at the posterior part of the spinal canal directly over to the dorsal column.
Results
From a general point of view, three factors of lasting success of SCS treatments have so far been identified: 1) an optimal selection of patients; 2) the exact coverage of the aching zone and 3) the quality of the followup controls. SCS must be considered a life-long treatment. In our practice, routine medical controls are performed by the same doctor at 3 weeks, 3, 6 and 12 months after implantation, and every year thereafter. Moreover patients are instructed to call the hospital if they experience any change in the perceived stimulation effects. For PAOD patients, the functional status of both limbs and the tcpO2 values in the supine and sitting positions are assessed at each control session. The stimulation parameters are routinely checked and, whenever necessary, modified. AP patients are simply requested to answer a standard questionnaire aiming to quantify the benefits and drawbacks of the treatment; the stimulation parameters are also readjusted if necessary. Furthermore, it has to be stressed that the outcomes of SCS can only be compared to those of conservative treatments. Comparisons with reconstructive techniques are not pertinent because SCS is still strictly reserved to patients in whom reconstructions have already failed or are considered not feasible. Moreover, reports on reconstructive procedures include a large amount of patients who either suffer from noncritical limb ischemia, in whom SCS has no proven benefit, or who are in the most severe stage of the disease, and, hence, beyond SCS treatment capabilities. Finally, the performance of blinded studies is hampered by the nature of the treatment, which requires the presence of evoked paresthesias in the aching area.
Atherosclerotic chronic critical limb ischemia
A systematic review [6,7] of controlled studies comparing SCS with any form of conservative treatment in patients with inoperable chronic critical limb ischemia (CCLI) found significantly higher limb salvage rates after 12 months, more pronounced pain relief and a significantly better chance of reaching Fontaine stage ll in SCS treated patients. Moreover, recent data from our group [8] indicate persistent benefits beyond the first year of treatment, as shown by a limb salvage rate of 78% after 5 years of SCS treatment in 24 long-term survivors. Strikingly, in this survey neither previous sympathectomy nor prostaglandin treatments influenced the outcome and SCS did not perform better in stage lll than in stage lV patients.
Non-atherosclerotic CCLI
Success rates above average have been reported in Raynaud and Buerger disease [8,9]. In contrast, heterogeneous data have been obtained in chronic regional pain syndromes (CRPS), which are characterised by both sensory and autonomic disturbances [8,10].
Refractory AP
Investigations from independent centres have evidenced substantial improvements in various aspects of the perceived quality of life; significant reduction of anginal complaints and of short-acting nitrates consumption, along with increases in exercise capacity and anginal threshold, were almost unanimously reported [11]. Accordingly, SCS has been recommended since 2002 by the European Society of Cardiology’s (ESC) joint study group for treating refractory AP [12].
The efficacy of SCS in AP is mostly supported by various randomised trials, one placebo-controlled study and several controlled trials [11]. Noteworthy, among 104 patients with triple vessel disease randomly assigned to either SCS treatment or to coronary artery bypass grafting (CABG), Mannheimer et al. [13] found 6 months postoperatively less mortality (p = 0.02) in SCS patients, and equivalent symptom relief in both groups, albeit better exercise capacity (p = 0.02) after CABG. Follow-up investigation of the same patients at 5 years found identical mortality in both groups [14]. In contrast, longitudinal studies have failed to demonstrate any influence of SCS treatment on left ventricular function [15] or cardiac arrhythmias [16]. Finally, the fear that SCS could deprive the patient of a vital warning signal and, thus favour the development of cardiac ischemia and myocardial infarction has vanished over time because all available data establish that SCS raises anginal threshold without eliminating the typical pain from AP or symptoms of myocardial infarction. Also, long-term studies including a large number of patients, have concluded that SCS neither adversely affects mortality nor morbidity [14,17,18].
Mechanisms of action
In PAOD
Multiple lines of evidence indicate that the beneficial effects of SCS on limb ischemia are mostly mediated by early changes in the microcirculation [19,20,21,22]. Serial microcirculatory investigations using intravital capillary microscopy, skin thermometry, isotopic techniques, laser Doppler fluxmetry and measurements of transcutaneous oxygen pressure (tcpO2) have demonstrated that SCS increases both the thermoregulatory and the nutritive skin blood flow substantially [20]. Strikingly, these effects are strictly limited to the distribution area of the stimulated spinal segments. The specific impact of SCS on the nutritive skin blood flow has been assessed in pioneering work by Jacobs et al. [19] who performed intravital capillary microscopy in 20 consecutive SCS responders and found significant increases in: a) capillary density (p <0.01); b) number of sodium fluorescein-perfused capillaries (p <0.001); c) peak red blood cell velocity (p <0.001); and d) a decrease in sodium fluorescein appearance time (p <0.001). These data have helped in closing an old debate on a pure placebo effect of SCS, as repeatedly advocated by its early opponents.
The neural mechanisms behind these microcirculatory changes have been an ongoing matter of discussion for nearly two decades. This discussion has mostly focused on three mechanisms proposed by Tallis et al. [23] in 1992. They have postulated that:
- a)
- the general pain-relieving effect of SCS leads secondarily to reversal of the sympathetic vasoconstriction, commonly elicited by painful sensations. This hypothesis is no longer tenable since it has been unequivocally demonstrated that vasodilatation always precedes, most often for days, pain alleviation [9,22];
- b)
- SCS reduces the sympathetic tone by a direct effect on spinal and supraspinal autonomic controls;
- c)
- antidromic stimulation of afferent nerve fibres leads to the release of vasoactive compounds at the peripheral nerve endings.
The last two proposals are supported by solid experimental data. In rats, the vasodilatory response to SCS is decreased after partial, and abolished after full, sympathectomy [24], indicating that sympathetic activity is necessary to mediate the vasodilatory effect of SCS. Moreover, later pharmacological studies on the same animal model and performed by the same group [24,25] have shown that nicotinic transmission in both the ganglia and postganglionic α1 receptors is deeply involved in SCS-induced peripheral dilatation.
The antidromic hypothesis has been initially rejected because of the common belief that the current intensities used in animal investigations could not be applied in the clinical settings. This opinion prevailed until it was finally demonstrated, at the end of the last century, that current intensities below discomfort level actually induce an antidromic activity in the dorsal roots leading to peripheral vasodilation [26,27,28,29]. Moreover, animal experiments have shown a strong reduction of the vasodilatory response to SCS in response to the administration of either the calcitonin gene-related peptide (CGRP) receptor antagonist CGRP-8-37 [27,28] or of the nitric oxide (NO) synthase inhibitor L-NAME [29]. Finally, available literature suggests a possible concomitant but still minor involvement of numerous other neuromediators such as vasoactive intestinal polypeptide (VIP), substance P, neuropeptide Y (NPY) γ-amino-butyric acid (GABA) or prostaglandins (30).
In refractory AP
Early publications on this topic have proposed that the beneficial effects of SCS on AP are mediated by a modulation of the autonomic nervous system. Over the last decades, however, this hypothesis has been progressively abandoned, mainly because neither heart rate nor heart rate variability are influenced in AP patients receiving SCS [16].
Three main mechanisms of action are presently thought to contribute to the beneficial effects of SCS on heart ischemia and anginal threshold. These are:
- −
- a reduction of oxygen myocardial consumption: a human study of 20 patients stressed with atrial pacing measured significantly less lactates in the coronary sinus at a comparable pacing rate under SCS [31];
- −
- a redistribution of the coronary blood flow towards the regions with impaired myocardial perfusion, as evidenced by positron emission tomography (PET) studies in 9 patients [32];
- −
- a more efficient cardiac work. This hypothesis stems from hemodynamic investigations in mini-pigs showing substantial decreases in systemic and pulmonary vascular resistances along with significant increases in cardiac output and stroke volume during low-intensity (2 volts) SCS applied at the highest possible cervical level [33].
Each of these mechanisms, that do not exclude each other, can account for, or participate to, the anti-ischemic effect of SCS in AP.

Table 1.
Peripheral arterial occlusive disease.
Table 1.
Peripheral arterial occlusive disease.
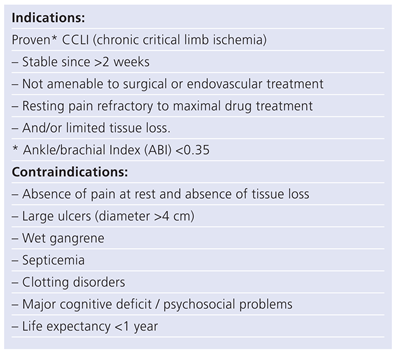 |

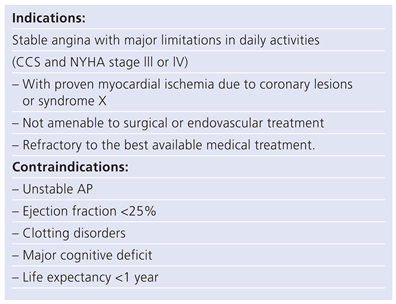
Table 2.
Angina pectoris.
Table 2.
Angina pectoris.
 |
Indications, contraindications and selection criteria
Indications and contraindications to SCS are summarized in tables 1 (PAOD) and 2 (AP).
Implanted pacemakers and defibrillators do not preclude SCS treatment, but special peroperative testing must be performed to avoid “cross-talking” between devices.
Selection criteria
In PAOD
Given that most skin lesions ultimately result more from maldistribution of cutaneous blood flow than from reduced total blood flow [34] and that the pain alleviating effect of SCS mostly results from local microcirculatory vasodilation [9,22] only targeted microcirculatory tests can provide data allowing accurate selection of potential treatment responders. Critical limb ischemia (CLI) results from multiple occlusions of microvessels along with vasospastic microcirculatory changes. Whilst occlusions are obviously irreversible, vasospastic changes can occur following SCS treatment. Hence, the most suitable – and at the present time unique – way to predict SCS outcome consists of a precise assessment of the vasospastic component. To reach this goal, various dynamic microcirculatory tests are available. Intravital capillary microscopy at rest and during reperfusion appears to be the most accurate way to assess the changes of nutritional blood flow to the skin in various conditions. Unfortunately, however, intravital capillary microscopy is time-consuming and requires complex equipment, as well as very special expertise [19]. Conversely, serial measurements of transcutaneous oxygen tension (tcpO2), either in the supine and sitting positions [35] or at rest and in the early phase of reperfusion [21], can be easily performed in almost every medical centre. Owing to their remarkably high predictive value, these simple and less resource-consuming tests are increasingly used to target possible treatment responders [21,35,36].
In refractory AP
Measuring local changes in the cardiac microcirculation is a complex task. Therefore, and in contrast to SCS for treatment of PAOD, there are no measurable variables allowing accurate predictions of responses to SCS in AP. At the present time, either transcutaneous electrical nerve stimulation (TENS) screening [37] or temporary SCS testing are mostly used to select potential responders to SCS treatment [11].
Drawbacks and complications of SCS treatment
The occurrence of spinal cord compression by epidural hematoma or infections with consecutive spinal abscess remain exceptional. In both cases, prompt recognition and treatment usually prevent permanent damage. Infection of any part of the device requires immediate withdrawal of the implanted material and antibiotics; in case of intrathecal bleeding, emergency neurosurgery must be considered. Neither centralised data nor written reports of permanent central nervous system complications due to SCS lead implantation have been published to date. Therefore, the fear of serious neurological damages following lead implantation is mostly founded on the experience of anesthesiologists, who roughly estimate that 1 out of 15 000 to 20 000 patients is at risk to develop an epidural hematoma after peridural anesthesia and that circa 30% to 50% of these patients will suffer late sequelas.
Lead infection rates of 0 to 3% have most often been reported in the recent literature. Despite both systematic antibiotic prophylaxis and the application of strictly sterile surgical techniques, the authors had to face this problem in 2 patients (0.8%). At the present time, lead infection rates of 1 to 1.5% seem acceptable.
Leakage of cerebrospinal fluid is another possible, albeit much less severe complication; it seldom requires specific treatment. A few reports also mention pain or discomfort at the site of spinal puncture or around the battery, as well as intolerance towards the implanted material. The necessity of re-interventions over time is a major drawback of SCS treatment. Migration of percutaneous leads occurs in 10 to 20% of cases and, in spite of technological advances, rupture in around 3%. These complications usually require surgical replacement of the implanted material [38].
Finally, SCS alleviates angina symptoms without masking the symptoms of impending acute myocardial infarction [11]. As a consequence, neither long-term mortality nor morbidity is adversely affected by SCS treatment.
Future developments
New technological developments will help reduce the number of reinterventions. The recent advent of rechargeable batteries has already allowed a reduction in the number of battery replacements.
High-quality trials are still necessary to improve the selection of SCS candidates and to demonstrate cost-effectiveness of SCS treatment. Moreover, the number of indications will rise. Isolated attempts to improve the run-off of high risk distal bypasses or to promote the healing of ischemic skin flaps have led to apparently promising results. Sparse data also indicate that several weeks of SCS could help lower the level of non-urgent vascular amputations [39]. Randomised prospective studies are urgently needed to assess the validity and cost-effectiveness of these new strategies.
Finally, growing experience with SCS could lead to repositioning SCS earlier in the treatment algorithm of selected PAOD and AP patients.
Conclusions
Surgical or endovascular treatment are clearly the best options for treating CCLI of the limbs and refractory AP. Therefore, SCS is definitely not an alternative to reconstructive procedures. However, it represents a particularly valuable mode of treatment in carefully selected patients in whom vascular reconstruction has failed, is not possible, or is too risky. Late treatment failures are very rare in both AP and CCLI.
SCS for critical heart and limb ischemia is much more than just pain therapy. It must be considered to be a true vascular treatment that requires a multidisciplinary approach, as well as the competence of skilled vascular and heart laboratories with special expertise in microcirculatory work.
Lack of information on the possibilities of SCS is responsible for non-referral of eligible patients. SCS, in contrast to vascular surgery, does not require any incision in ischemic tissue, is fully reversible and is moderately invasive. Routine microcirculatory screening can be advised in most non-reconstructable CCLI patients. TENS or a SCS trial period of several days should also be proposed to refractory AP patients for maximal treatment.
Conflicts of Interest
No conflict of interest to disclose.
References
- Melzack, R.; Wall, P. Pain mechanisms: a new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.W.; Oygar, A.; Baggenstos, P.; et al. Vascular disease of the extremities: Electrical stimulation of spinal cord and posterior roots. NY State J Med. 1976, 76, 366–368. [Google Scholar]
- Murphy, D.; Giles, K. Dorsal column stimulation for pain relief from intractable angina pectoris. Pain 1987, 28, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Holsheimer, J. Computer modeling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord. 1998, 36, 531–540. [Google Scholar] [CrossRef]
- Holsheimer, J.; Struijk, J.J. How do geometric factors influence epidural spinal cord stimulation?Aquantitative analysis by computer modeling. Stereotact Funct Neurosurg. 1991, 56, 234–249. [Google Scholar] [CrossRef]
- Ubbink, D.T.; Vermeulen, H. Spinal cord stimulation for non-reconstructable chronic critical limb ischemia (Cochrane review). In The Cochrane Library; Oxford, 2003; Issue 3; Update Software. [Google Scholar]
- Amann, W.; Berg, P.; Gersbach, P.; et al. for the SCS-EPOS Study Group. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischemia: Results of the European peripheral vascular disease outcome study (SCS-EPOS). Eur J Vasc Endovasc Surg. 2003, 26, 280–286. [Google Scholar] [CrossRef]
- Gersbach, P.A.; Argitis, V.; Gardaz, J.-P.; von Segesser, L.K.; Haesler, E. Late outcome of spinal cord stimulation for unreconstructable and limbthreatening lower limb ischemia. Eur J Vasc Endovasc Surg. 2007, 33, 717–24. [Google Scholar] [CrossRef][Green Version]
- Spincemaille, G.H. Spinal cord stimulation in peripheral vascular disease. In Pain Research and Clinical Management; Electrical stimulation and the relief of pain; Simpson, B.A., Ed.; Elsevier, 2003; Volume 15, pp. 131–142, chap 9. [Google Scholar]
- Bennett, D.S.; Cameron, T.L. Spinal cord stimulation for complex regional pain syndromes. In Pain Research and Clinical Management; Electrical stimulation and the relief of pain; Simpson, B.A., Ed.; Elsevier, 2003; Volume 15, pp. 110–129, chap 8. [Google Scholar]
- Andréll, P.; Yu, W.; Gillberg, L.; et al. Long-term effects of spinal cord stimulation in refractory angina pectoris—3 year results from the EuropeanAngina Registry Link (EARL). Abstract book ESC Congress: Barcelona, 2008. [Google Scholar]
- Mannheimer, C.; Camici, P.; Chester, M.R.; et al. The problem of chronic refractory angina pectoris; report from the ESC Joint Study Group on the treatment of refractory angina. Eur Heart J. 2002, 23, 355–370. [Google Scholar] [CrossRef]
- Mannheimer, C.; Eliasson, T.; Augustinsson, L.E.; et al. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris. The ESBY study. Circulation. 1998, 97, 1157–1163. [Google Scholar] [CrossRef]
- Ekre, O.; Eliasson, T.; Norsell, H.; et al. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study. Eur Heart J. 2002, 23, 1938–1945. [Google Scholar] [CrossRef][Green Version]
- Hautvast, R.W.; Szabo, B.M.; DeJongste, M.J.L.; et al. Influence of spinal cord stimulation on left ventricular function in patients with refractory angina pectoris. In Proceedings of the 2nd International Symposium on Heart Failure-mechanisms and management; 1993. [Google Scholar]
- Eliasson, T.; Augustinsson, L.E.; Mannheimer, C. Spinal cord stimulation in severe angina pectoris – presentation of current studies, indications, and clinical experience. Pain 1996, 65, 169–179. [Google Scholar] [CrossRef]
- Greco, S.; Auriti, A.; Fiume, D; et al. Spinal cord stimulation for the treatment of refractory angina pectoris: a two-year follow-up. PACE 1999, 22, 26–32. [Google Scholar] [CrossRef]
- Ten Vaarwerk, I.; Jessurun, G.; de Jongste, M.; et al. Clinical outcome of patients treated with spinal cord stimulation for therapeutically refractory angina pectoris. Heart 1999, 82, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.J.H.M.; Jörning, P.J.G.; Beckers, R.C.Y.; et al. Foot salvage and improvement of microvascular blood flow as a result of epidural spinal cord electrical stimulation. J Vasc Surg. 1990, 12, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Gersbach, P. Microcirculatory screening for spinal cord stimulation. Crit Ischemia 1999, 9, 39–45. [Google Scholar]
- Gallay, D. Spinal cord stimulation for the treatment of peripheral vascular disease: patients selection: clinical and haemodynamic criteria. In Spinal Cord Stimulation for Peripheral Vascular Diseases; Herreros, J, Lazorthes, Y., Galley, D., Broggi, G., Eds.; ELA Madrid, 1994; Advances and controversies. [Google Scholar]
- Meyerson, B.A.; Linderoth, B. Spinal cord stimulation: mechanisms of action in neuropathic and ischaemic pain. In: Pain Research and Clinical Management, vol 15, BA Simpson ed. Electrical stimulation and the relief of pain, Elsevier 2003, chap 11, pp. 161–182.
- Tallis, R.; Jacobs, M.; Miles, J. Spinal cord stimulation in peripheral vascular disease (Editorial). Br J Neurosurg. 1992, 6, 101–105. [Google Scholar] [CrossRef]
- Linderoth, B.; Gunasekera, L.; Meyerson, B.A. Effects of sympathectomy on skin and muscle microcirculation during dorsal column stimulation: animal studies. Neurosurgery 1991, 29, 874–879. [Google Scholar] [CrossRef]
- Linderoth, B.; Herregodts, P. Meyerson BA. Sympathetic mediation of peripheral vasodilation induced by spinal cord stimulation:Animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery 1994, 15, 711–719. [Google Scholar] [CrossRef]
- Croom, J.E.; Barron, K.W.; Chandler, M.J.; Foreman, R.D. Cutaneous blood flow increases in the rat hindpaw during dorsal column stimulation. Brain Res. 1996, 728, 281–286. [Google Scholar] [CrossRef]
- Croom, J.E.; Foreman, R.D.; Chandler, M.J.; Barron, K.W. utaneous vasodilatation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am J Physiol. 1997, 272, H950–H957. [Google Scholar]
- Tanaka, S.; Barron, K.W.; Chandler, M.J.; et al. Low intensity spinal cord stimulation may induce cutaneous vasodilation via CGRP release. Brain Res. 2001, 896, 183–187. [Google Scholar] [CrossRef]
- Croom, J.E.; Foreman, R.D.; Chandler, M.J.; et al. Role of nitric oxide in cutaneous blood flow increases in the rat hindpaw during dorsal column stimulation. Neurosurgery 1997, 40, 565–571. [Google Scholar] [PubMed]
- Linderoth, B. Dorsal column stimulation and pain. Experimental studies of putative neurochemical and neurophysiological mechanisms. Thesis Stockholm 1992. [Google Scholar]
- Mannheimer, C.; Eliasson, T.; Anderson, B.; et al. ffects of spinal cord stimulation in angina pectoris induced by pacing and possible mechanisms of action. Br Med J. 1993, 307, 477–480. [Google Scholar] [CrossRef]
- Hautvast, R.W.; Blanksma, P.K.; DeJongste, M.J.; et al. Effect of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol. 1996, 77, 462–477. [Google Scholar] [CrossRef]
- Gersbach, P.A.; Hasdemir, M.G.; Eeckhout, E.; von Segesser, L.K. Spinal cord stimulation treatment for angina pectoris: more than a placebo? Ann Thorac Surg. 2001, 72, S1–100. [Google Scholar] [CrossRef]
- Claeys, L.G.Y.; Horsch, S. Effects of spinal cord stimulation on ischemia inflammatory pain and wound healing in patients with peripheral arterial occlusive disease Fontaine stage lV. Pain Digest. 1997, 7, 200–203. [Google Scholar]
- Gersbach Ph Hasdemir, M.G.; Stevens, R.D.; Nachbur, B.; Mahler, F. Discriminative microcirculatory screening of patients with refractory limb ischemia for dorsal column stimulation. Eur J Vasc Endovasc Surg. 1997, 13, 464–471. [Google Scholar] [CrossRef]
- Ubbink, D.T.; Tulveski, I.I.; de Graaff, J.C.; Legemate, D.A.; Jacobs, M.J.H.M. Optimisation of the non-invasive assessment of critical limb ischemia requiring invasive treatment. Eur J Vasc Endovasc Surg. 2000, 19, 131–7. [Google Scholar] [CrossRef]
- Mannheimer, C.; Carlsson, C.A.; Emanuelsson, H.; et al. The effects of transcutaneous electrical nerve stimulation in patients with severe angina pectoris. Circulation. 1985, 71, 308–316. [Google Scholar] [CrossRef]
- Spincemaille, G.H.; Klomp, H.L.; Steyerberg, E.W.; et al. Technical data and complications of of spinal cord stimulation: data from a randomized trial on critical limb ischemia. Stereotact Funct Neurosurgery. 2000, 74, 63–72. [Google Scholar] [CrossRef]
- Gersbach, P.; Gardaz, J.P.; Ferrari, E.; et al. La stimulation électrique épidurale pour maladie vasculaire périphérique et coronarienne. Résultas et perspectives. Rev Med Suisse. 2008, 150, 797–804. [Google Scholar]
© 2010 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
