Inflammation and Stroke †
Summary
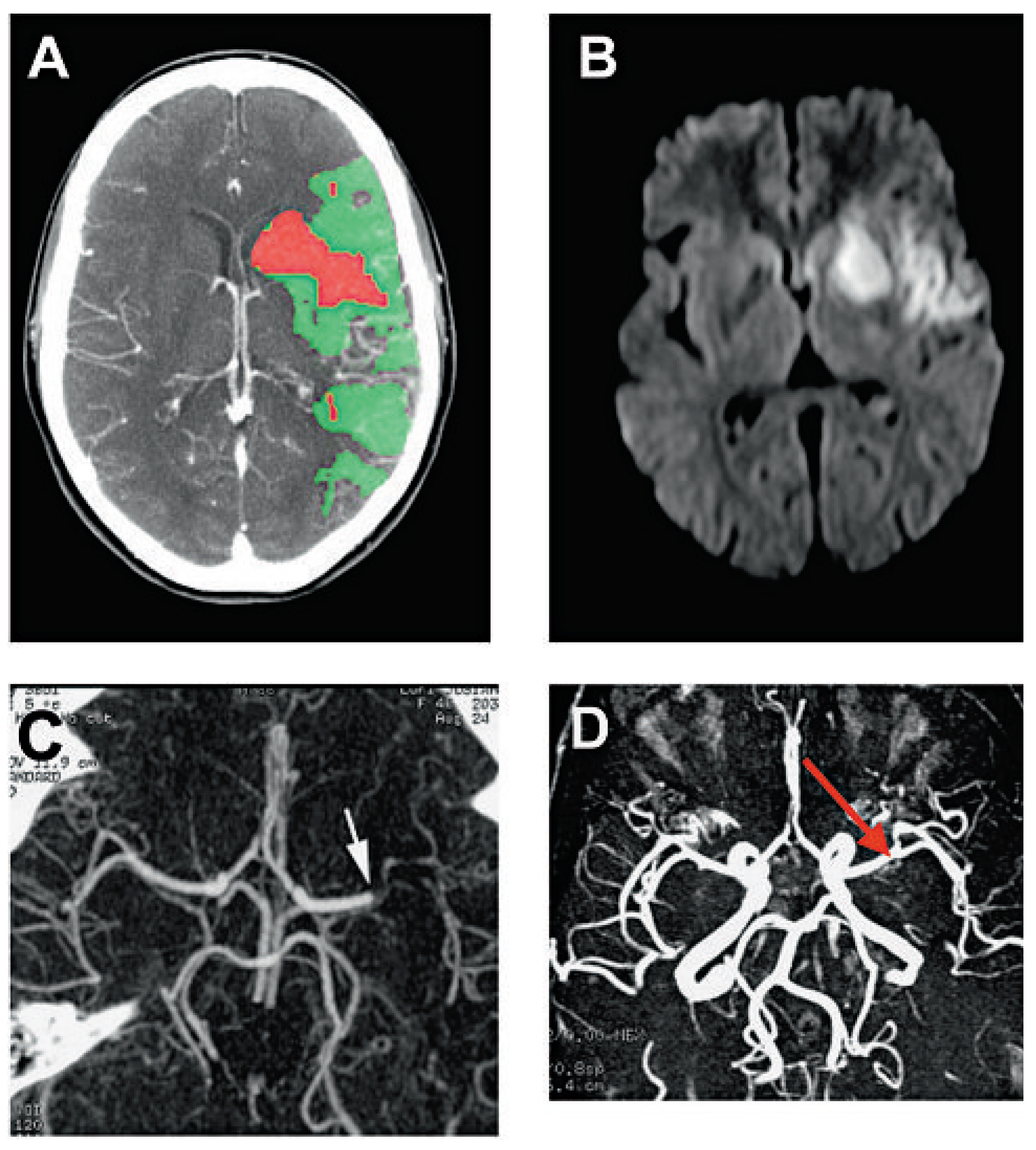
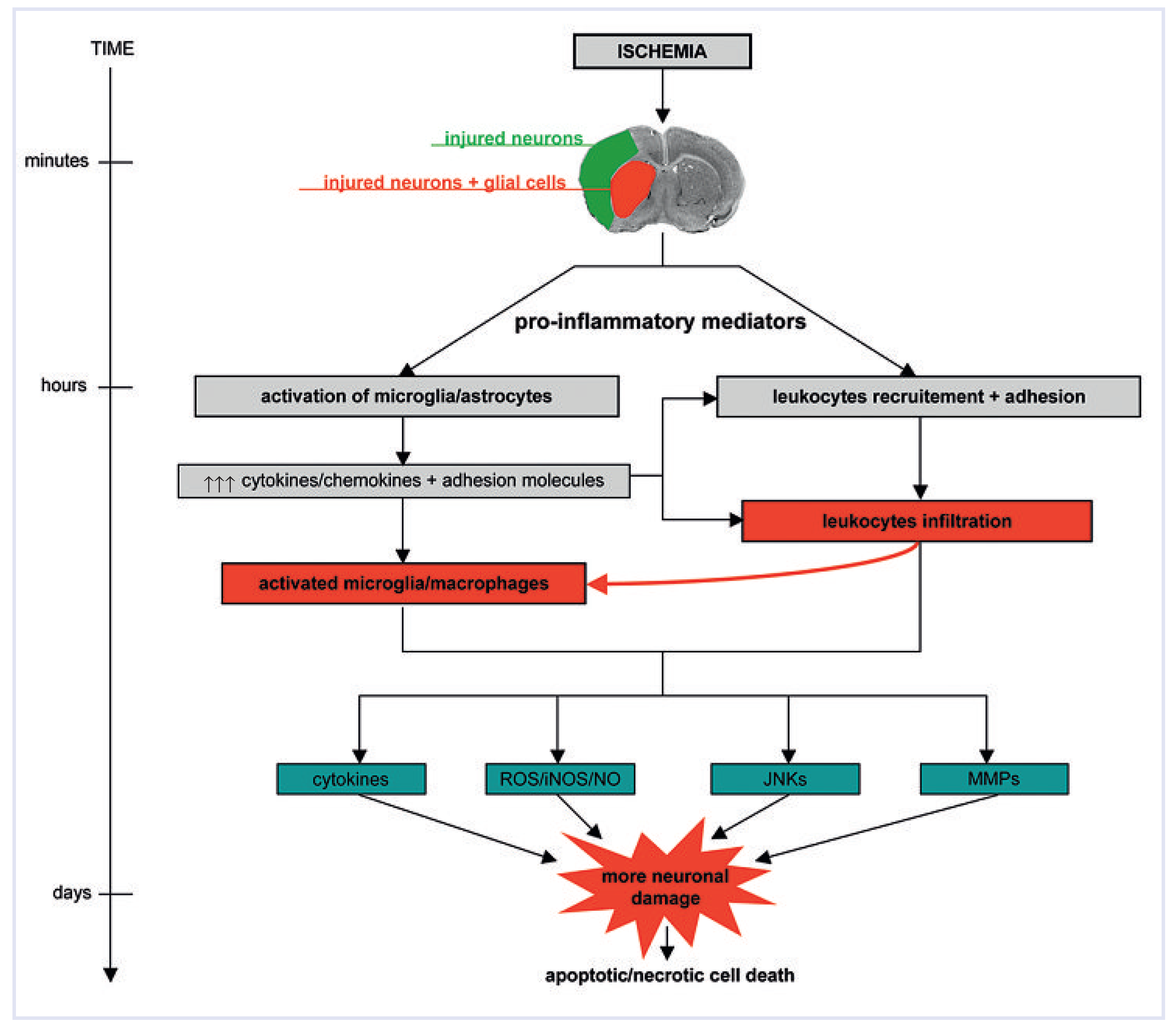
Neuroinflammatory response after ischaemia
 |
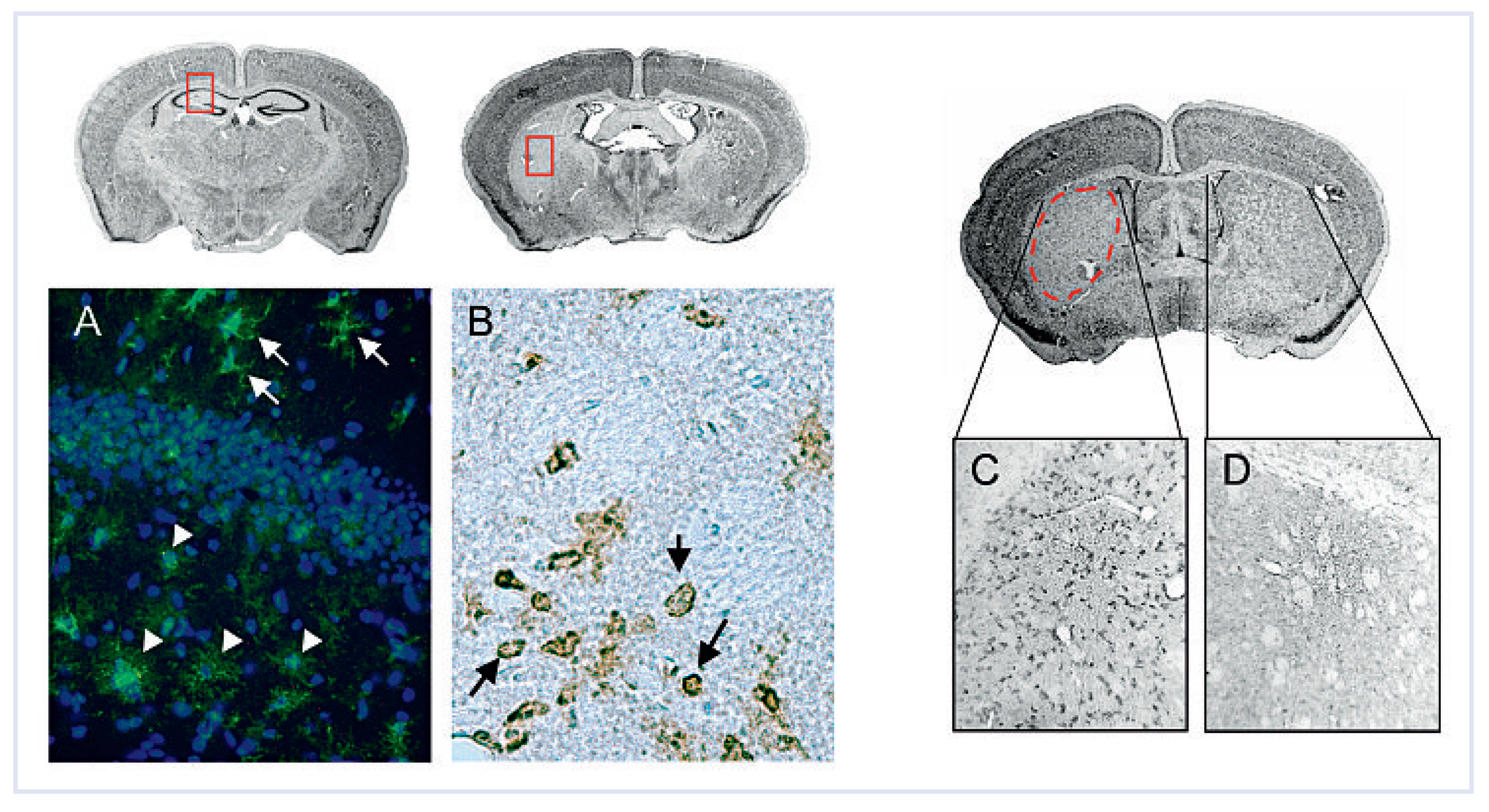
Glial cell activation
Leukocyte infiltration
 |
Transcriptional regulation of inflammation
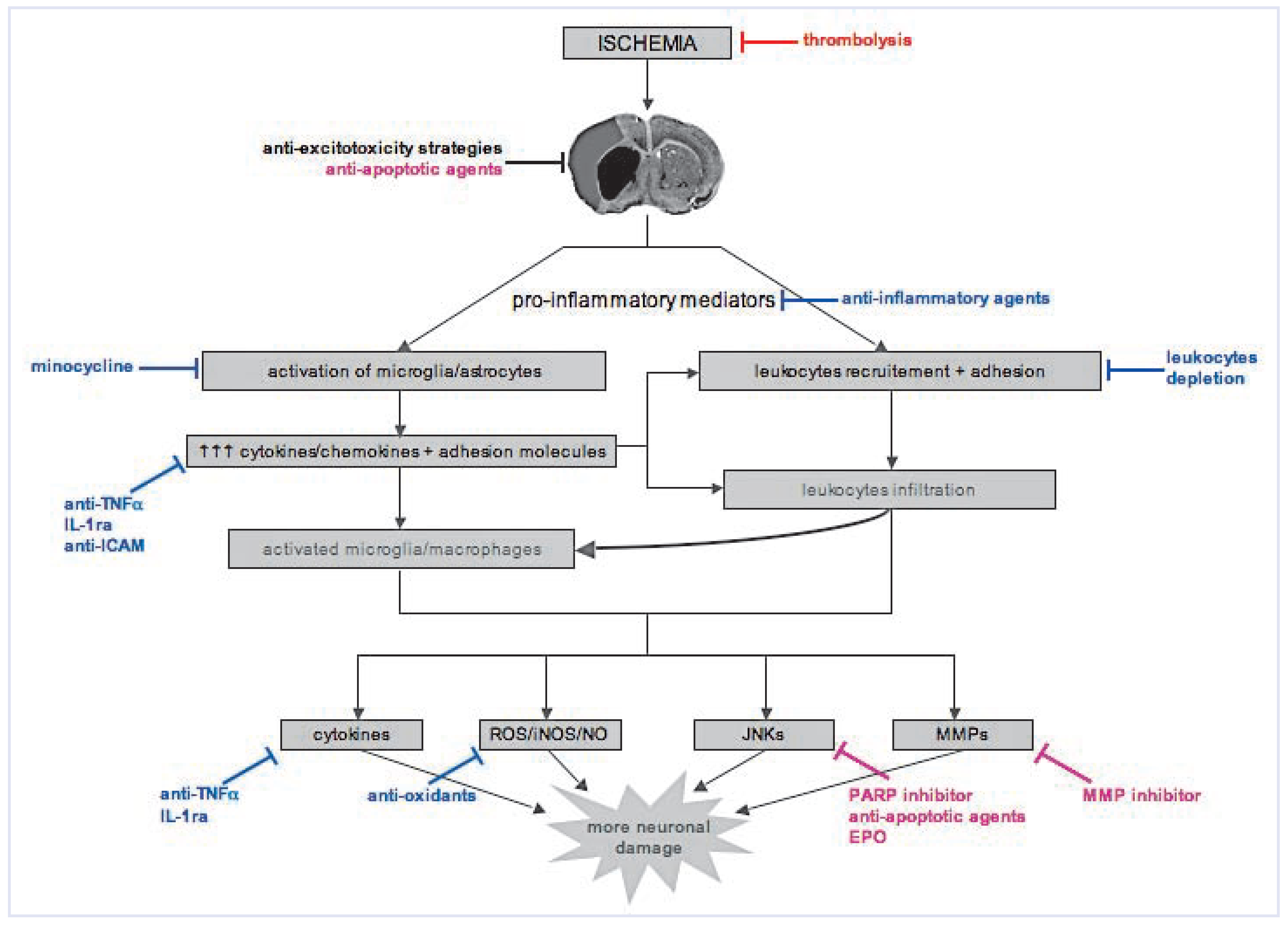
Neuroprotective approaches targeting inflammation
Experimental studies to clinical trials: lost in translation
Autoimmune and infectious aetiologies of stroke
Funding
Acknowledgments
References
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Mehta, S.L.; Manhas, N.; Raghubir, R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res. Rev. 2007, 54, 34–66. [Google Scholar] [CrossRef] [PubMed]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Higashida, R.; Wechsler, L.; Gent, M.; Rowley, H.; Kase, C.; et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA 1999, 282, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997, 28, 2109–18. [Google Scholar] [CrossRef]
- Nicole, O.; Docagne, F.; Ali, C.; Margaill, I.; Carmeliet, P.; MacKenzie, E.T.; et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signalling. Nat. Med. 2001, 7, 59–64. [Google Scholar] [CrossRef]
- Wiegler, K.; Bonny, C.; Coquoz, D.; Hirt, L. The JNK inhibitor XG-102 protects from ischemic damage with delayed intravenous administration also in the presence of recombinant tissue plasminogen activator. Cerebrovasc. Dis. 2008, 26, 360–366. [Google Scholar] [CrossRef]
- Barone, F.C.; Feuerstein, G.Z. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J. Cereb. Blood Flow. Metab. 1999, 19, 819–834. [Google Scholar] [CrossRef]
- del Zoppo, G.J.; Becker, K.J.; Hallenbeck, J.M. Inflammation after stroke: is it harmful? Arch. Neurol. 2001, 58, 669–672. [Google Scholar] [CrossRef]
- Petty, M.A.; Wettstein, J.G. Elements of cerebral microvascular ischaemia. Brain Res. Brain Res. Rev. 2001, 36, 23–34. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, X.N.; Yenari, M.A. The inflammatory response in stroke. J. Neuroimmunol. 2007, 184, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Zhang, J.H.; Nanda, A.; Granger, D.N. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front. Biosci. 2004, 9, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- del Zoppo, G.J.; Ginis, I.; Hallenbeck, J.M.; Iadecola, C.; Wang, X.; Feuerstein, GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000, 10, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, G.Z.; Wang, X.; Barone, F.C. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation 1998, 5, 143–59. [Google Scholar] [CrossRef]
- Raivich, G.; Bohatschek, M.; Kloss, C.U.; Werner, A.; Jones, L.L.; Kreutzberg, G.W. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res. Brain Res. Rev. 1999, 30, 77–105. [Google Scholar] [CrossRef]
- Ladeby, R.; Wirenfeldt, M.; Garcia-Ovejero, D.; Fenger, C.; Dissing-Olesen, L.; Dalmau, I.; et al. Microglial cell population dynamics in the injured adult central nervous system. Brain Res. Brain Res. Rev. 2005, 48, 196–206. [Google Scholar] [CrossRef]
- Streit, W.J.; Walter, S.A.; Pennell, N.A. Reactive microgliosis. Prog. Neurobiol. 1999, 57, 563–581. [Google Scholar] [CrossRef]
- Garden, G.A.; Moller, T. Microglia biology in health and disease. J. Neuroimmune Pharmacol. 2006, 1, 127–137. [Google Scholar] [CrossRef]
- Hanisch, U.K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Bezzi, P.; Domercq, M.; Brambilla, L.; Galli, R.; Schols, D.; De, C.E.; et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 2001, 4, 702–710. [Google Scholar] [CrossRef]
- Kimelberg, H.K. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia 2005, 50, 389–397. [Google Scholar] [CrossRef]
- del Zoppo, G.J.; Hallenbeck, JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb. Res. 2000, 98, 73–81. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Mehra, A.; Connolly, E.S., Jr.; D‘Ambrosio, A.L. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: a critical review of the literature. Inflamm. Res. 2004, 53, 497–508. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Deng, Q.; Yao, H.H.; Wang, D.C.; Deng, Y.; Liu, G.Q. Inducible nitric oxide synthase expression in the ischemic core and penumbra after transient focal cerebral ischemia in mice. Life Sci. 2002, 71, 1985–1996. [Google Scholar] [CrossRef]
- Iadecola, C.; Forster, C.; Nogawa, S.; Clark, H.B.; Ross, M.E. Cyclooxygenase2 immunoreactivity in the human brain following cerebral ischemia. Acta Neuropathol. 1999, 98, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997, 20, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernandez-Guillamon, M.; Lo, E.H.; Montaner, J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Stephenson, D.; Yin, T.; Smalstig, E.B.; Hsu, M.A.; Panetta, J.; Little, S.; et al. Transcription factor nuclear factor-kappa B is activated in neurons after focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 2000, 20, 592–603. [Google Scholar] [CrossRef]
- Kunz, A.; Abe, T.; Hochrainer, K.; Shimamura, M.; Anrather, J.; Racchumi, G.; et al. Nuclear factor-kappaB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J. Neurosci. 2008, 28, 1649–1658. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK 14 pathways as molecular targets for antiinflammatory therapy – from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Borsello, T.; Clarke, P.G.; Hirt, L.; Vercelli, A.; Repici, M.; Schorderet, D.F.; et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 2003, 9, 1180–1186. [Google Scholar] [CrossRef]
- Hirt, L.; Badaut, J.; Thevenet, J.; Granziera, C.; Regli, L.; Maurer, F.; et al. DJNKI1, a cell-penetrating c-Jun-N-terminal kinase inhibitor, protects against cell death in severe cerebral ischemia. Stroke 2004, 35, 1738–43. [Google Scholar] [CrossRef] [PubMed]
- Waetzig, V.; Czeloth, K.; Hidding, U.; Mielke, K.; Kanzow, M.; Brecht, S.; et al. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 2005, 50, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Hidding, U.; Mielke, K.; Waetzig, V.; Brecht, S.; Hanisch, U.; Behrens, A.; et al. The c-Jun N-terminal kinases in cerebral microglia: immunological functions in the brain. Biochem. Pharmacol. 2002, 64, 781–788. [Google Scholar] [CrossRef]
- Iadecola, C.; Alexander, M. Cerebral ischemia and inflammation. Curr. Opin. Neurol. 2001, 14, 89–94. [Google Scholar] [CrossRef]
- Emsley, H.C.; Smith, C.J.; Georgiou, R.F.; Vail, A.; Hopkins, S.J.; Rothwell, N.J.; et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1366–1372. [Google Scholar] [CrossRef]
- O‘Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar]
- Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 2001, 57, 1428–1434. [CrossRef]
- Lees, K.R.; Zivin, J.A.; Ashwood, T.; Davalos, A.; Davis, S.M.; Diener, H.C.; et al. NXY-059 for acute ischemic stroke. N. Engl. J. Med. 2006, 354, 588–600. [Google Scholar] [CrossRef]
- Dirnagl, U. Bench to bedside: the quest for quality in experimental stroke research. J. Cereb. Blood Flow. Metab. 2006, 26, 1465–1478. [Google Scholar] [CrossRef]
- Chamorro, A.; Hallenbeck, J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke 2006, 37, 291–293. [Google Scholar] [CrossRef]
- Ringleb, P.A.; Strittmatter, E.I.; Loewer, M.; Hartmann, M.; Fiebach, J.B.; Lichy, C.; et al. Cerebrovascular manifestations of Takayasu arteritis in Europe. Rheumatology 2005, 44, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Cantini, F.; Boiardi, L.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. N. Engl. J. Med. 2002, 347, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Brown, R.D.; Jr Calamia, K.T.; Christianson, T.J.; Weigand, S.D.; Miller, D.V.; et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann. Neurol. 2007, 62, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Bobba, R.S.; Johnson, S.R.; Davis, A.M. A review of the sapporo and revised Sapporo criteria for the classification of antiphospholipid syndrome. Where do the revised sapporo criteria add value? J. Rheumatol. 2007, 34, 1522–1527. [Google Scholar]
- Bart, P.A. When should consider antiphospholipid syndrome? Rev. Med. Suisse 2008, 4, 97–99. [Google Scholar]
- Lim, W.; Crowther, M.A.; Eikelboom, J.W. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006, 295, 1050–1057. [Google Scholar] [CrossRef]
- Dirnagl, U.; Klehmet, J.; Braun, J.S.; Harms, H.; Meisel, C.; Ziemssen, T.; et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke 2007, 38 (Suppl. S2), 770–773. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Reichhart, M.; Thiran, J.P.; Maeder, P.; Chalaron, M.; Schnyder, P.; et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann. Neurol. 2002, 51, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidou, C.; Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002, 1, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Zhang, F.; Xu, X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am. J. Physiol. 1995, 268 Pt 2, R286–R292. [Google Scholar] [CrossRef][Green Version]
- Parmentier, S.; Bohme, G.A.; Lerouet, D.; Damour, D.; Stutzmann, J.M.; Margaill, I.; et al. Selective inhibition of inducible nitric oxide synthase prevents ischaemic brain injury. Br. J. Pharmacol. 1999, 127, 546–552. [Google Scholar] [CrossRef]
- Veldhuis, W.B.; Floris, S.; van der Meide, P.H.; Vos, I.M.; de Vries, H.E.; Dijkstra, C.D.; et al. Interferon-beta prevents cytokine-induced neutrophil infiltration and attenuates blood-brain barrier disruption. J. Cereb. Blood Flow. Metab. 2003, 23, 1060–1069. [Google Scholar] [CrossRef]
- Yang, G.Y.; Gong, C.; Qin, Z.; Ye, W.; Mao, Y.; Bertz, A.L. Inhibition of TNFalpha attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport 1998, 9, 2131–2134. [Google Scholar] [CrossRef]
- Yrjanheikki, J.; Tikka, T.; Keinanen, R.; Goldsteins, G.; Chan, P.H.; Koistinaho, J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. USA 1999, 96, 13496–13500. [Google Scholar] [CrossRef]
- Asahi, M.; Wang, X.; Mori, T.; Sumii, T.; Jung, J.C.; Moskowitz, M.A.; et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001, 21, 7724–7732. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Lai, Q.; Li, J.; Gordon, V.; Diaz, F.G. Long-term neuroprotective effect of inhibiting poly(ADP-ribose) polymerase in rats with middle cerebral artery occlusion using a behavioral assessment. Brain Res. 2001, 915, 210–217. [Google Scholar] [CrossRef]
- Moroni, F. Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage. Curr. Opin. Pharmacol. 2008, 8, 96–103. [Google Scholar] [CrossRef]
- Endres, M.; Namura, S.; Shimizu-Sasamata, M.; Waeber, C.; Zhang, L.; Gomez-Isla, T.; et al. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J. Cereb. Blood Flow. Metab. 1998, 18, 238–247. [Google Scholar] [CrossRef]
- Hara, H.; Friedlander, R.M.; Gagliardini, V.; Ayata, C.; Fink, K.; Huang, Z.; et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 2007–2012. [Google Scholar] [CrossRef]
- Ehrenreich, H.; Hasselblatt, M.; Dembowski, C.; Cepek, L.; Lewczuk, P.; Stiefel, M.; et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 2002, 8, 495–505. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2009 by the author. Attribution - Non-Commercial - NoDerivatives 4.0
Share and Cite
Benakis, C.; Hirt, L.; Du Pasquier, R.A. Inflammation and Stroke. Cardiovasc. Med. 2009, 12, 143. https://doi.org/10.4414/cvm.2009.01424
Benakis C, Hirt L, Du Pasquier RA. Inflammation and Stroke. Cardiovascular Medicine. 2009; 12(5):143. https://doi.org/10.4414/cvm.2009.01424
Chicago/Turabian StyleBenakis, Corinne, Lorenz Hirt, and Renaud A. Du Pasquier. 2009. "Inflammation and Stroke" Cardiovascular Medicine 12, no. 5: 143. https://doi.org/10.4414/cvm.2009.01424
APA StyleBenakis, C., Hirt, L., & Du Pasquier, R. A. (2009). Inflammation and Stroke. Cardiovascular Medicine, 12(5), 143. https://doi.org/10.4414/cvm.2009.01424




