Summary
The investigation of recurrent unexplained syncope is a challenging clinical problem, as syncope remains unexplained in up to 60% of patients referred to an emergency department. In spite of the demonstrated benefit of a standardised stepwise non invasive work-up, syncope still remains undiagnosed in up to 30% of patients. The “gold standard” for the diagnostic work-up of syncope is the correlation between clinical event and any alteration of physiological signals, but syncope rarely occurs during ECG monitoring. Recent advances in long-term cardiac monitoring techniques with implantable loop recorders (ILR) offer nowadays a powerful tool for the investigation of syncope as well as undiagnosed arrhythmias.
According to current indications, ILR is recommended in patients with recurrent unexplained syncope following a negative baseline work-up, who require further investigations because of syncope-related complications. ILR can also be implanted early in the work-up, before conventional investigations, in patients with clinical or ECG features suggesting an arrhythmic syncope or to assess the contribution of bradycardia in suspected cases of severe neurally mediated syncope before pacemaker implantation. Recently, ILR was proposed as a novel method for atrial fibrillation monitoring, particularly after catheter ablation, but it deserves additional clinical validation due to a high prevalence of inappropriate detection.
Key words: implantable loop recorder; unexplained syncope; current indications; diagnostic yield; atrial fibrillation inappropriate detection
Introduction
Syncope is a relatively common clinical symptom accounting for 3–6% of all emergency department visits and 2% of hospital admissions [
1,
2]. Despite multiple investigations, syncope remains unexplained in up to 60% of patients referred to an emergency department [
3,
4]. Superior performance of standardised investigation strategies has been shown in patients addressed to emergency departments for unexplained syncope [
3,
5,
6]. Iglesias et al. recently published [
7] in >900 consecutive patients referred to a syncope outpatient clinic that a stepwise work-up established an aetiology in 66% and that 92% of diagnoses were determined combining non invasive tests. Invasive investigations poorly contributed to overall diagnostic yield (8%), stressing usefulness of non invasive testings. In spite of the benefit of a standardised work-up, syncope still remains undiagnosed in up to 30% of patients. Ideally, correlation between syncopal events and any alteration of physiological signals (ECG, blood pressure, EEG, etc.) is the “gold standard” for the diagnostic work-up, but syncope rarely occurs during monitoring.
Holter monitoring and external loop recorders (ELR) have been available for many years. According to ESC Guidelines [
8], Holter monitoring is indicated (class I recommendation) in patients with clinical or ECG features suggesting an arrhythmia and very frequent episodes (≥1/week). ELR permits extended rhythm monitoring and ECG storage before and after the clinical event; however, technical restrictions limit their use to relatively short periods (10 days) making the recording of a spontaneous event and its correlation with cardiac arrhythmias unlikely. Therefore, overall diagnostic yield of Holter monitoring in the evaluation of syncope is relatively poor, accounting for 9% in a retrospective review [
9]. Structural heart disease, impaired ejection fraction and advanced age were found to be significant predictors of a diagnostic Holter study, suggesting that short-term ECG monitoring results highly depend on patients’ selection. Similarly, Sarasin et al. [
10] reported a diagnostic rate of Holter monitoring in unselected patients with unexplained syncope as low as 6%, increasing to 12% when restricted to high-risk patients with positive cardiac history or abnormal ECG.
Deception and challenges made some investigators to modify a pacemaker to make it a subcutaneous recorder without cardiac leads. Recent advances in long-term cardiac monitoring techniques with implantable loop recorders (ILR) offer nowadays a powerful tool for the investigation of syncope and undiagnosed arrhythmias.
The Implantable Loop Recorder
Indications
The ILR is a single channel ECG monitoring device developed to extend the monitoring period beyond 1 month in patients with unexplained syncope. According to ESC guidelines, the ILR is recommended in patients with recurrent unexplained syncope following a negative baseline work-up, who require further investigations because of complications. It can also be implanted early before any conventional testing in patients with clinical or ECG features suggesting an arrhythmic syncope or to assess contribution of bradycardia in suspected cases of severe neurally mediated syncope before pacemaker implantation (class II recommendations). Recently, the ILR has also been evaluated for atrial fibrillation (AF) monitoring before and after ablation procedures [
11,
12].
Technical Characteristics
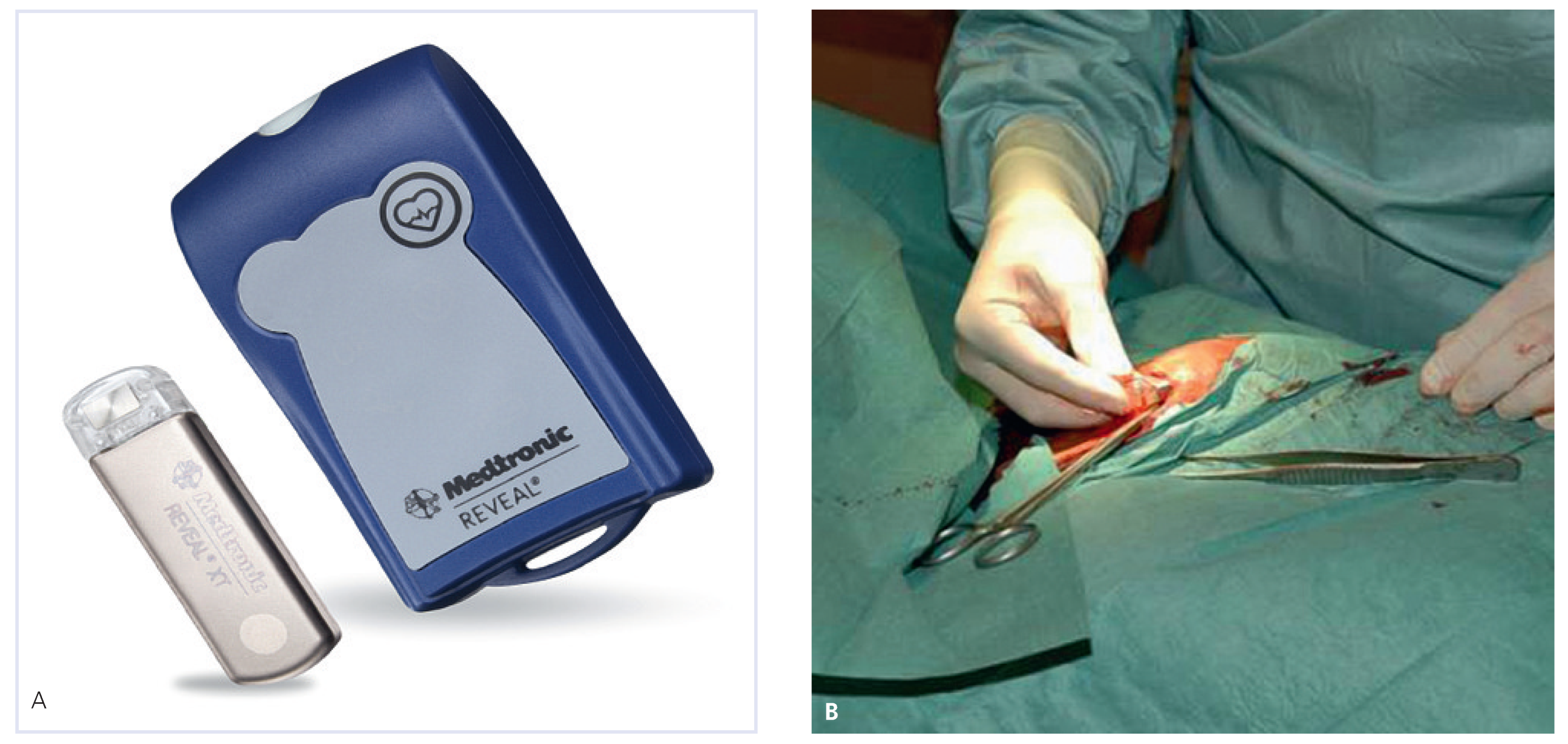
The latest version of the device (Reveal DX and XT®, Medtronic Inc., USA) is rectangular, measures 62 × 19 × 8 mm and weighs 17 grams (
Figure 1). A pair of sensing electrodes built in the shell records a single-lead bipolar ECG, retrievable by radiofrequency with a programmer. The battery has an estimated lifetime of 36 months. The device is inserted into subcutaneous tissue of the left pectoral region after an incision of approximately 2 cm in length under local anaesthesia. Other locations include right parasternal, subcostal and axillary areas providing, nevertheless, a signal of lower amplitude. ECG signal is stored in a circular buffer with a maximal storage capacity of 49.5 min (up to 30 events) allowing detection of pauses as well as high and low heart rate episodes. The XT version has an algorithm for atrial tachycardia and AF detection based on irregularities of RR intervals. Events are automatically stored (1 min for asystole, bradycardia or ventricular tachycardia (VT), 2 min for atrial tachycardia or AF) (
Figure 2). The ECG memory buffer can also be frozen using a hand-held activator after syncope has resolved (6 min before and 1.5 min after activation for the 3 last episodes). However, the ILR bears some limitations related to overdetection of inappropriate arrhythmias and underdetection of potentially malignant arrhythmias in the auto-activation mode. Documented causes of incorrect arrhythmia storage include oversensing related to sudden reductions in R wave amplitude during normal sinus rhythm and arrhythmias, undersensing by transient loss of ECG signal because of device amplifier saturation and oversensing related to T wave, myopotentials or other noise sources. Recently, a new ILR (SJM Confirm®, St. Jude Medical, Inc., USA) of similar size has been commercialised. Its diagnostic performances have not been compared with the Medtronic-ILR yet.
Evidence from the Literature
Initial Experience with the ILR
In 1995, Krahn et al. reported the first experience with an ILR [
13]. In a pilot study including 16 patients with recurrent unexplained syncope, a pacemaker-based prototype was implanted following a negative baseline work-up including ambulatory continuous ECG monitoring, cardiac imaging, head-up tilt-testing (HUTT) and electrophysiological study (EPS). Over a mean follow-up of 4 months, 15 patients (94%) presented a syncopal event; an arrhythmia was diagnosed in 60%. In another study [
14] including 24 patients with unexplained syncope, a prototype was implanted after a negative baseline work-up; among the 20 patients (83%) who experienced syncope during follow-up, an aetiology was established in all cases, including arrhythmias in 50% and non arrhythmic causes in 50%. Of note, individualised therapy resulted in resolution of symptoms in 85% of patients with recurrence, emphasising the diagnostic and therapeutic potential of this new technology. A few years later, a multicenter prospective study [
15] evaluated the benefit of a prolonged monitoring strategy in 85 patients with recurrent unexplained syncope after a negative initial evaluation. Over 11 months of follow-up, symptoms recurred in 68%; interestingly, an arrhythmia was diagnosed in 42%, providing evidence for effectiveness and safety of an ILR-based prolonged monitoring strategy. Later on, Seidl et al. [
16] reported the results of a multicenter study including 133 patients with recurrent unexplained syncope. The setting of this study did not include any standardised work-up. Over a mean follow-up of 11 months, 62% of patients presented syncope, establishing a symptom-rhythm correlation in 87% of cases with recurrence; overall diagnostic yield of ILR reached 54%. Again, an arrhythmia was diagnosed in 44%.
Table 1 displays summary data of the most important studies that used the ILR for the investigation of unexplained syncope, including our experience at the CHUV syncope outpatient clinic. On the basis of these studies, overall diagnostic yield of ILR is about 50%. However, these results need to be cautiously interpreted because diagnostic yield of ILR highly depends upon patients’ selection and adjudication of final diagnoses. Patients’ selection may differ depending on baseline characteristics of study population (e.g., presence of structural heart disease) and patient’s inclusion criteria. Some studies excluded patients with typical clinical presentation of neurally mediated syncope [
21,
28] or with indication for pacemaker implantation following a positive HUTT or carotid sinus massage (CSM) [
24], decreasing consequently diagnostic yield of conventional testings. Diagnostic rate also appears overestimated due to inclusion of normal sinus rhythm as diagnostic during syncope recurrence. Exclusion of an arrhythmic cause cannot be considered as diagnostic, as sinus rhythm at time of recurrence may be associated with a broad spectrum of aetiologies such as neurally mediated syncope, psychogenic pseudo-syncope and hypotensive or neurological disorders. Proportion of sinus rhythm considered as diagnostic is about 20% for an average diagnostic yield of about 50%. Although some authors have considered the ILR as the gold standard for syncope management, an unexpectedly high proportion of patients (5–10%) failed to activate the device. The newer ILR generation has eased the handheld activation, which should decrease failure rate.
ILR as an Alternative to Conventional Testing
Krahn et al. [
21] randomised 66 patients with unexplained syncope to conventional testing (ELR, HUTT, EPS) or to 1-year monitoring with an ILR. A final diagnosis was established in 52% of patients investigated with an ILR, while conventional work-up yielded a diagnosis in only 20%. Importantly, superiority of the ILR-based strategy over conventional testing had been strengthened by excluding patients whose cause was likely vasovagal. Results might have been different for unselected patients. Benefit of prolonged monitoring strategy was also suggested in another study [
22] including 206 patients implanted with an ILR because of unexplained syncope after a negative conventional work-up including HUTT and EPS. After 6 months, overall diagnostic yield of ILR achieved 64%, including 23% of arrhythmias. Farwell et al. [23, 24] compared the use of ILR with conventional testings for evaluation of 201 consecutive patients without structural heart disease presenting to an emergency department for recurrent unexplained syncope. Eligible patients underwent a baseline investigation including CSM and HUTT; those without indication for pacemaker implantation were randomized to either conventional testing (Holter monitoring, ELR, EPS) or ILR implantation. Diagnostic rate was again significantly higher in ILR patients as compared to conventionally investigated patients (43% vs 7%, respectively) over 17 months of follow-up. Moreover, patients in the ILR arm underwent fewer post-randomisation investigations and hospitalisation days. Time to second syncope was significantly longer in the ILR arm with subsequent reduction in syncopal events and improvement in quality of life. No statistically significant difference in overall mortality (12%) and global costs savings was noted between the two investigation strategies. Nevertheless, as stated above, patients were highly selected, preventing any conclusion about usefulness of ILR in an unselected population.
In a recent prospective study by Pezawas et al. [
25], an ILR was implanted in 70 patients with recurrent unexplained syncope following a negative baseline investigation including CSM, HUTT, echocardiogram and Holter monitoring. Interestingly, half of the patients (47%) had documented structural heart disease. Syncope recurrence rate during follow-up was similar in patients with and without structural heart disease (about 85%); an arrhythmia was documented in half of the cases in both groups. Interestingly, presence of a major depressive disorder was predictive of early syncopal recurrence, while documented structural heart disease was poorly predictive of arrhythmias. Finally, in the ISSUE-2 study [
26], 392 patients with >2 episodes of suspected neurally mediated syncope were implanted with an ILR and followed until the first documented recurrence. Patients with significant ECG or cardiac abnormalities, orthostatic hypotension and carotid sinus hypersensitivity had been excluded. Recurrence occurred in 33% after a year of follow-up. The ILR provided an ECG tracing in only 26% because 7% failed to activate the device. Patients with recurrence underwent an ILR-based therapy including pacemaker and defibrillator implantation, catheter ablation, antiarrhythmic drug or no specific therapy when no arrhythmia had been detected at time of recurrence. The 1-year syncope recurrence was significantly lower in the ILR-based therapy arm compared with patients without specific therapy (10% vs 41%, respectively). Authors concluded that an ILR-based strategy and therapy was a reasonable alternative to usual management of patients with suspected neurally mediated syncope. However, treatment groups had not been randomised, preventing any conclusions on the therapeutic potential of the ILR.
Unexpected Therapeutic Properties of Implantable Devices
A placebo effect of implantable devices on syncope recurrence has been well documented over the last decade. In the VPS II trial [
27], 100 patients with recurrent HUTT-positive syncope were implanted with a dual chamber pacemaker and randomised to inactive (ODO) or active (DDD) pacing mode. Over a follow-up of 6 months, syncope recurred only in 42% and 33% of active and inactive groups, respectively, suggesting that implantation itself dramatically reduced syncope recurrence. Significant reduction in syncope rate is also a common finding following ILR implantation. Nierop et al. [
28] reported resolution of symptoms in 17% after ILR implantation. In the different substudies of the ISSUE trial, patients with a mean of ≥2 syncopal episodes during the last 2 years prior to randomisation experienced a decrease in syncope recurrence by a factor of 2 [
18], 3 [
17] and 5 [
19], respectively, in the year following ILR implantation. Moreover, 2/
3 of ILR-implanted patients included in the ISSUE 2 trial [
26], with a median of 4 syncopal events during the last 2 years prior to implantation, did not experience recurrence over a median follow-up of 9 months, highlighting the potential placebo effect of ILR in patients suffering from syncope. The way that ILR potential placebo effect may affect overall diagnostic rate of ILR studies needs to be evaluated by future formal randomised placebo-controlled studies.
Cost-Effectiveness of ILR
One trial [
29] evaluated the cost implications of a prolonged monitoring strategy in the investigation of unexplained syncope. 60 patients with unexplained syncope were randomised to either conventional testing (ELR, HUTT, EPS) or 1-year ILR monitoring. The latter yielded a diagnosis in 47%, while conventional testing established a diagnosis in 20%. Although the cost was greater in the ILR monitoring group compared with conventional testing group, cost per diagnosis was significantly reduced because of the greater diagnostic yield, suggesting superiority in term of cost-effectiveness of a primary ILR-based strategy. However, some limitations have to be mentioned as patients with suspected vasovagal syncope or left ventricular dysfunction had been excluded, preventing any conclusion about the poor diagnostic yield of HUTT and EPS.
ILR in Pediatric Population
Studies about the use of ILR in paediatric patients are limited. No consensual guidelines are currently available, but ILR is recommended after a negative conventional work-up in children with structural heart disease, family history of sudden death, symptoms associated with exercise, chest pain, palpitations or ECG suggesting an arrhythmia [
8]. In one study [
31], the diagnostic yield of ILR reached 50%. However, ILR showed some limitations because of an imperfect automatic algorithm leading to episodes of auto-activation failure as well as frequent false-positive activations related to muscle artefacts that may have over-recorded arrhythmias. In another recent study [
32], ILR implantation showed unexpected therapeutic properties, leading to resolution of symptoms in about 50% of implanted patients.
Experience at the CHUV Syncope Outpatient Clinic
The CHUV syncope outpatient clinic investigated over the last 9 years >1000 patients referred for unexplained syncope [
7]. Patients underwent a standardised work-up including HUTT followed by supine and upright CSM. Structural heart disease was ruled out on the basis of patient’s history, physical examination and 12-lead ECG. EPS was performed in patients with an underlying cardiac disease or in patients whose initial non invasive evaluation was negative, and who required further investigations. An ILR was proposed to 136 patients following a negative work-up because of syncope-related complications; 57 patients accepted implantation (5% of total population). 6 additional patients with an indication other than syncope were also implanted. Patients had a mean age of 58 ± 18 years and 35% were women. Ischaemic heart disease was documented in 6%. Sudden syncope was the presentation mode in 38%. Patients were followed up over a mean of 483 ± 287 days after ILR implantation. 1 patient was lost to follow-up.
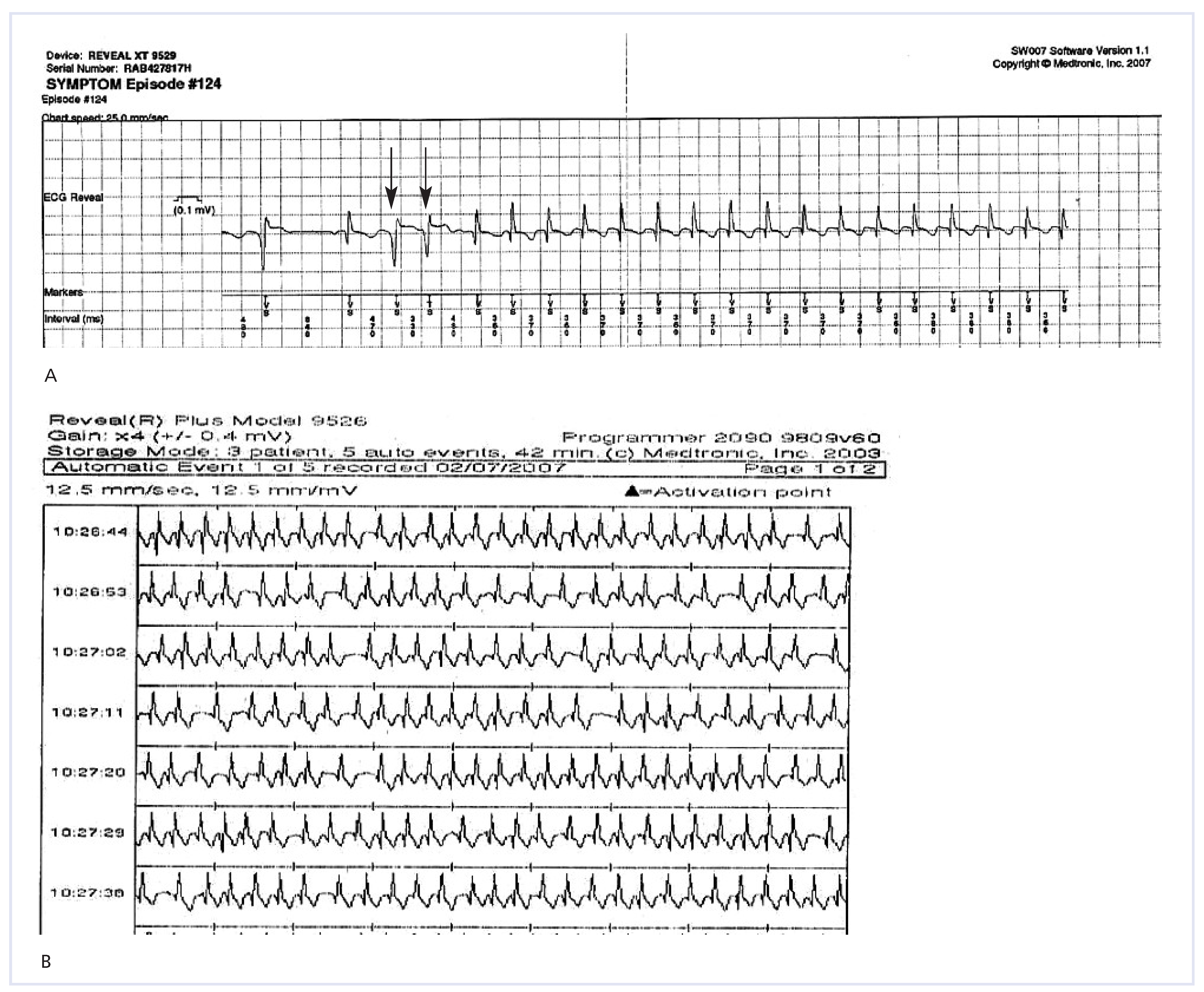
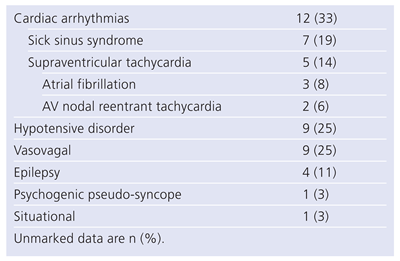
Table 2 shows the final causes of syncope as established by the ILR. Overall, the ILR yielded a diagnosis in 57% of patients, while syncope remained unexplained in 43%. An arrhythmia was diagnosed in 33%, with equal proportion of sick sinus syndrome (19%) and supraventricular tachycardia (14%). Interestingly, the ILR helped to diagnose intermittent hypotensive disorders in 25%. Remaining causes included epilepsy (11%), situational (3%) and psychogenic pseudo-syncope (3%). It is noteworthy that 2 among 4 of the patients diagnosed with supraventricular arrhythmias had undergone a negative EPS prior to ILR implantation. Similarly, 3 patients diagnosed with epilepsy had a negative neurological evaluation including electroencephalogram before implantation. These results confirm the incremental benefit of ILR implantation in patients with unexplained syncope after a negative standardised work-up. Our experience with newer generation of ILR also tends to confirm the current limitations of the AF detection algorithm based on irregularities of RR intervals.
Figure 3 shows examples of inappropriate detection of AF. These limitations emphasise the need for further technical improvements to make ILR a gold standard for AF diagnosis.
Conclusion
Growing evidence tends to support the incremental benefit of ILR in the investigation of recurrent unexplained syncope. The mean diagnostic yield of ILR reported in the literature is about 50–60% but strongly depends upon study settings and patients’ inclusion criteria. When limited to selected patients with severe unexplained syncope after a complete baseline non invasive work-up, our own experience confirmed these findings with a diagnosis yield of 57%. Whether the ILR may become the gold standard for the diagnosis of AF and follow-up of patients undergoing catheter ablation needs additional clinical validation. However, preliminary results report a high prevalence of inappropriate detection limiting reliability of the device to date.