Role of the Renin-Angiotensin-Aldosterone System in Diastolic Dysfunction and Heart Failure †
Summary
Introduction
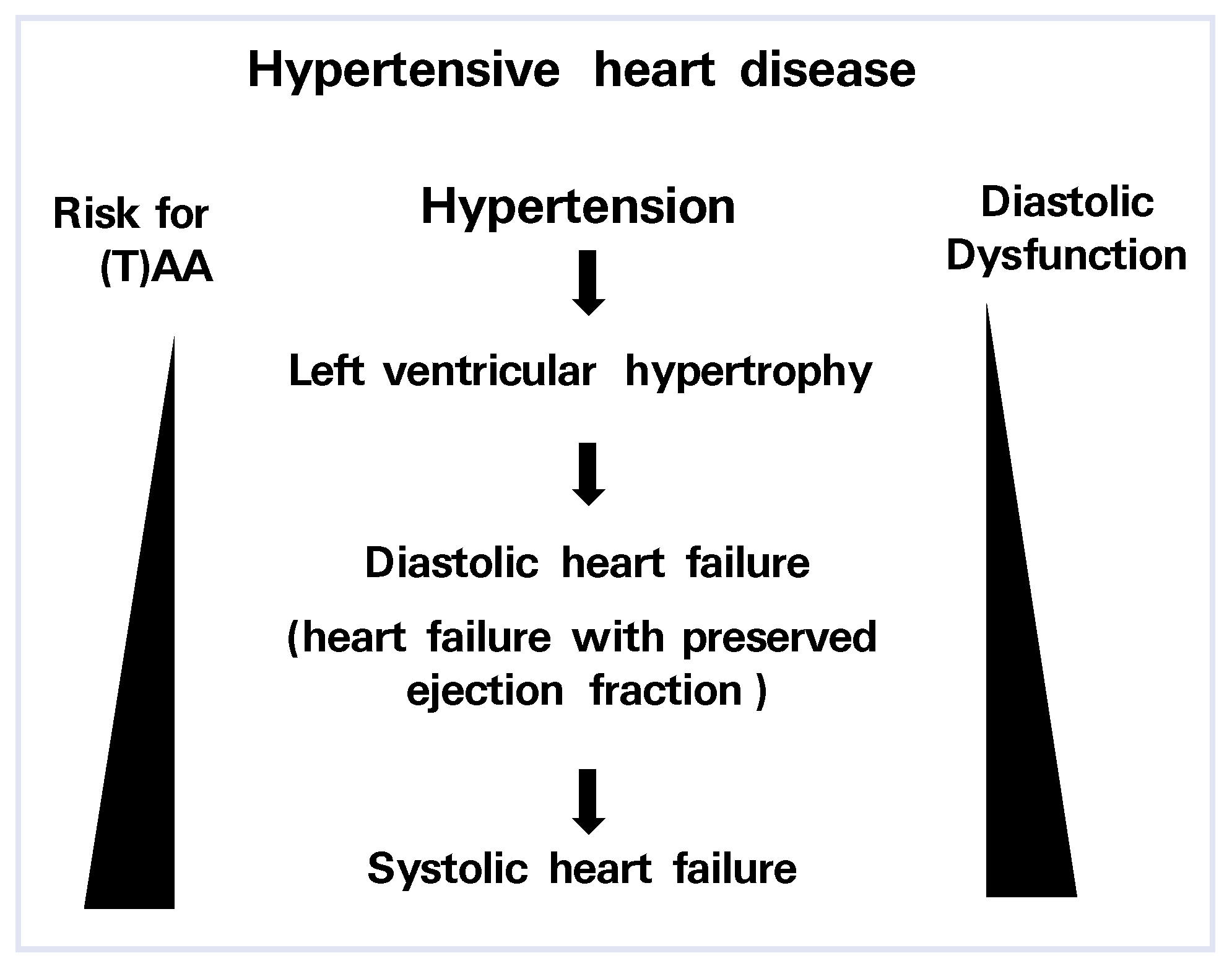
Hypertensive Heart Disease
RAAS Inhibition and LV Hypertrophy
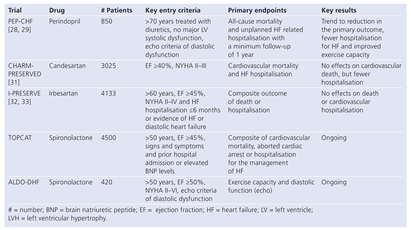
Outcome Studies in Patients with Heart Failure with Preserved Ejection Fraction
Conflicts of Interest
References
- Jessup, M.; Brozena, S. Heart failure. N Engl J Med. 2003, 348, 2007–2018. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Desnos, M.; Delahaye, F.; Emeriau, J.P.; Hanania, G.A. national survey of heart failure in French hospitals. The Myocardiopathy and Heart Failure Working Group of the French Society of Cardiology, the National College of General Hospital Cardiologists and the French Geriatrics Society. Eur Heart J. 2000, 21, 763–769. [Google Scholar] [CrossRef]
- Angeja, B.G.; Grossman, W. Evaluation and management of diastolic heart failure. Circulation. 2003, 107, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Johnson, G. Heart failure with normal ejection fraction. The V-HeFT Study. VeteransAdministration Cooperative Study Group. Circulation. 1990, 81, III48–III53. [Google Scholar]
- Vasan, R.S.; Benjamin, E.J.; Levy, D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. JAm Coll Cardiol. 1995, 26, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S.; Larson, M.G.; Benjamin, E.J.; Evans, J.C.; Reiss, C.K.; Levy, D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a populationbased cohort. J Am Coll Cardiol. 1999, 33, 1948–1955. [Google Scholar] [CrossRef]
- Smith, G.L.; Masoudi, F.A.; Vaccarino, V.; Radford, M.J.; Krumholz, H.M. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003, 41, 1510–1518. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006, 355, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C.; Fujiwara, K.; Lehoux, S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007, 117, 568–575. [Google Scholar] [CrossRef]
- Hill, J.A.; Olson, E.N. Cardiac plasticity. N Engl J Med. 2008, 358, 1370–1380. [Google Scholar] [CrossRef]
- Janardhanan, R.; Daley, W.L.; Naqvi, T.Z.; Mulvagh, S.L.; Aurigemma, G.; Zile, M.; et al. Rationale and design: the VALsartan In Diastolic Dysfunction (VALIDD) Trial: evolving the management of diastolic dysfunction in hypertension. Am Heart J. 2006, 152, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Janardhanan, R.; Verma, A.; Bourgoun, M.; Daley, W.L.; Purkayastha, D.; et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007, 369, 2079–2087. [Google Scholar] [CrossRef]
- Levy, D. Left ventricular hypertrophy. Epidemiological insights from the Framingham Heart Study. Drugs. 1988, 35, 51–55. [Google Scholar] [CrossRef]
- Klingbeil, A.U.; Schneider, M.; Martus, P.; Messerli, F.H.; Schmieder, R.E. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003, 115, 41–46. [Google Scholar] [CrossRef]
- Verdecchia, P.; Schillaci, G.; Borgioni, C.; Ciucci, A.; Gattobigio, R.; Zampi, I.; et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998, 97, 48–54. [Google Scholar] [CrossRef]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005, 112, e154–235. [Google Scholar]
- Swedberg, K.; Cleland, J.; Dargie, H.; Drexler, H.; Follath, F.; Komajda, M.; et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005, 26, 1115–1140. [Google Scholar] [CrossRef]
- Brilla, C.G.; Matsubara, L.; Weber, K.T. Advanced hypertensive heart disease in spontaneously hypertensive rats. Lisinopril-mediated regression of myocardial fibrosis. Hypertension. 1996, 28, 269–275. [Google Scholar] [CrossRef]
- Brilla, C.G.; Funck, R.C.; Rupp, H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000, 102, 1388–1393. [Google Scholar] [CrossRef]
- Schwartzkopff, B.; Brehm, M.; Mundhenke, M.; Strauer, B.E. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension 2000, 36, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Dahlof, B.; Devereux, R.B.; Kjeldsen, S.E.; Julius, S.; Beevers, G.; de Faire, U.; et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002, 359, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Baranchuk, A.; Crystal, E.; Morillo, C.A.; Garfinkle, M.; Yusuf, S.; et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005, 45, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Wachtell, K.; Lehto, M.; Gerdts, E.; Olsen, M.H.; Hornestam, B.; Dahlof, B.; et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: The Losartan Intervention For End point reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005, 45, 712–719. [Google Scholar] [CrossRef]
- Madrid, A.H.; Bueno, M.G.; Rebollo, J.M.; Marin, I.; Pena, G.; Bernal, E.; et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002, 106, 331–336. [Google Scholar] [CrossRef]
- Pitt, B.; Reichek, N.; Willenbrock, R.; Zannad, F.; Phillips, R.A.; Roniker, B.; et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4Eleft ventricular hypertrophy study. Circulation. 2003, 108, 1831–1838. [Google Scholar] [CrossRef]
- Solomon, S.D.; Appelbaum, E.; Manning, W.J.; Verma, A.; Berglund, T.; Lukashevich, V. et al. Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009, 119, 530–537. [Google Scholar] [CrossRef]
- Neutel, J.M.; Smith, D.H.G.; Weber, M.A. Effect of antihypertensive monotherapy and combination therapy on arterial distensibility and left ventricular mass. Am J Hypertension. 2004, 17, 37–42. [Google Scholar] [CrossRef]
- Cleland, J.G.; Tendera, M.; Adamus, J.; Freemantle, N.; Gray, C.S.; Lye, M.; et al. Perindopril for elderly people with chronic heart failure: the PEPCHF study. The PEP investigators. Eur J Heart Fail. 1999, 1, 211–217. [Google Scholar] [CrossRef]
- Cleland, J.G.; Tendera, M.; Adamus, J.; Freemantle, N.; Polonski, L. ; Taylor JThe perindopril in elderly people with chronic heart failure (PEPCHF) study. Eur Heart, J. 2006, 27, 2338–2345. [Google Scholar] [CrossRef]
- Tribouilloy, C.; Rusinaru, D.; Leborgne, L.; Peltier, M.; Massy, Z.; Slama, M. Prognostic impact of angiotensin-converting enzyme inhibitor therapy in diastolic heart failure. Am J Cardiol. 2008, 101, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Pfeffer, M.A.; Swedberg, K.; Granger, C.B.; Held, P.; McMurray, J.J.; et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003, 362, 777–781. [Google Scholar] [CrossRef]
- McMurray, J.J.; Carson, P.E.; Komajda, M.; McKelvie, R.; Zile, M.R.; Ptaszynska, A. et al. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008, 10, 149–156. [Google Scholar]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. RandomizedAldactone Evaluation Study Investigators. N Engl J Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Duprez, D.A.; Bauwens, F.R.; De Buyzere, M.L.; De Backer, T.L.; Kaufman, J.M.; Van Hoecke, J.; et al. Influence of arterial blood pressure and aldosterone on left ventricular hypertrophy in moderate essential hypertension. Am J Cardiol. 1993, 71, 17A–20A. [Google Scholar] [CrossRef] [PubMed]
- Mottram, P.M.; Haluska, B.; Leano, R.; Cowley, D.; Stowasser, M.; Marwick, T.H. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004, 110, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Guder, G.; Bauersachs, J.; Frantz, S.; Weismann, D.; Allolio, B.; Ertl, G.; et al. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007, 115, 1754–1761. [Google Scholar] [CrossRef]
 |
© 2009 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Bauersachs, J.; Widder, J.D. Role of the Renin-Angiotensin-Aldosterone System in Diastolic Dysfunction and Heart Failure. Cardiovasc. Med. 2009, 12, 74. https://doi.org/10.4414/cvm.2009.01403
Bauersachs J, Widder JD. Role of the Renin-Angiotensin-Aldosterone System in Diastolic Dysfunction and Heart Failure. Cardiovascular Medicine. 2009; 12(3):74. https://doi.org/10.4414/cvm.2009.01403
Chicago/Turabian StyleBauersachs, Johann, and Julian D. Widder. 2009. "Role of the Renin-Angiotensin-Aldosterone System in Diastolic Dysfunction and Heart Failure" Cardiovascular Medicine 12, no. 3: 74. https://doi.org/10.4414/cvm.2009.01403
APA StyleBauersachs, J., & Widder, J. D. (2009). Role of the Renin-Angiotensin-Aldosterone System in Diastolic Dysfunction and Heart Failure. Cardiovascular Medicine, 12(3), 74. https://doi.org/10.4414/cvm.2009.01403




