Case report
A 64-year-old woman was admitted because of fatigue and exertional dyspnoea, which had been worsening over one month. 26 years ago, radio-iodine thyroid ablation for diffuse autonomic hyperthyroidism had been performed. She had stopped taking thyroid hormone substitution ten years ago without consulting a physician. She had not regularly followed-up with her physician in the past ten years.
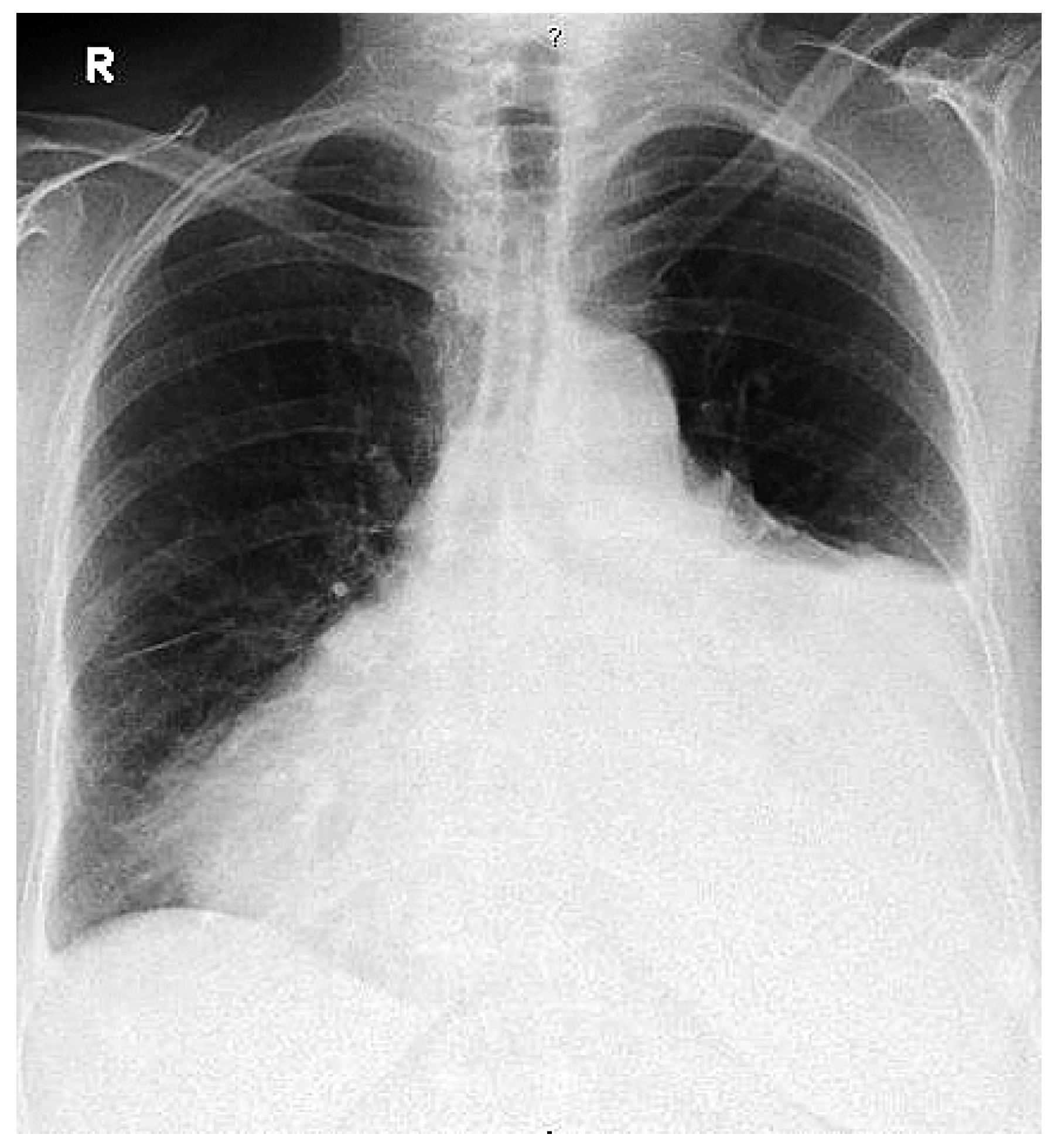
On admission she was dyspneic and orthopneic. Physical examination revealed mild malleolar oedema, a pericardial rub, fine basal pulmonary rales, normal heart sounds and no jugular venous distention. Blood pressure was 180/105 mm Hg, heart rate 80/min and temperature 36.3 °C. White blood cell count was 7.8 × 106/ml3 (36% band forms, 48% segmented forms, 11% lymphocyte, 4% monocyte, 1% basophile), thrombocyte count 579 × 106/ml3, haemoglobin 12.1 g/dl (erythrocyte indices within normal range). Significant biochemical results were: C-reactive protein 145 mg/l, alkaline phosphatase 148 U/l, creatine kinase 279 U/l, troponin T 0.11 ng/ml and Brain-Natriuretic-Peptide 196 pg/ml. Thyrotropin level was 12.41 mU/l (reference 0.25–5.0 mU/l), T3 level <0.4 pmol/l (reference 4.0–8.3 pmol/l) and T4 level <0.3 pmol/l (7.5–21.1 pmol/l). Antinuclear antibodies were 1:160 (reference <1:10). Normal serum values were found for creatinine, glucose (5.8 mmol/l), sodium potassium, ASAT, LDH (329 mmol/l) and total cholesterol. Rheumatoid factors, anti-neutrophil cytoplasmic antibodies, C3/C4 complement factors and CD4-/CD8-lymphocyte counts were within normal ranges. Electrocardiogram showed a regular sinus rhythm, small septal R and unspecific T-changes. A chest x-ray revealed a large cardiac silhouette, large left hemi thoracic effusion and blunting of the right costophrenical angle (fig. 1). CT scan of the chest showed massive pericardial effusion and some pleural effusion (fig. 2) bilaterally. Cardiac tamponade was ruled out by echocardiogram. 600 ml of straw coloured pericardial fluid was percutaneously drained. The fluid contained 8.0 × 106 leucocytes/ml3 (34% neutrophils, 56.5% lymphocytes). Glucose level was 2.7 mmol/l, LDH 3380 U/l, and total protein was 50.3 g/l. Repeated blood cultures remained sterile. However, cultures of pericardial effusion grew Salmonella enteritidis. Oral thyroxine substitution and antibiotic therapy with ciprofloxacin was started, the latter was maintained for a total of two weeks with progressive clinical and radiologic improvement. On follow-up six weeks after admission, the patient was in good condition and an echocardiogram showed only minimal pericardial effusion.
Discussion
Hypothyroidism is a well known cause of pericardial effusion. In the past, its occurrence detected by echocardiography was reported to be as high as 30 to 80% in patients with untreated hypothyroidism [
1]. However, these patients had evident clinical features of myxoedema and therefore cannot be compared with hypothyroid patients, who are diagnosed and treated early because thyroid function tests are readily available in modern medicine. In this population, pericardial effusion seems to be rare: Kabadi et al. reported an incidence of only 3 to 6% [
2].
The extent of pericardial effusions seems to be related to the severity and the duration of hypothyroidism. Amounts of up to 6000 ml of pericardial effusion in hypothyroidism have been reported [
2].
In our case, hypothyroidism was not severe, so that the extent of the pericardial effusion is surprising. However, the fluid may accumulate slowly in mild disease as in our patient, who had not been under thyroid hormone substitution for about ten years. The slow course of hypothyroidism may have allowed large quantities of effusion to accumulate due to the distensibility of the pericardial sac, thus evading cardiac tamponade [
3]. This mechanism may explain the absence of clinical signs or echocardiographic evidence of haemodynamic compromise in our patient.
Percutaneous drainage guided by echocardiography is the therapy of choice in large effusions. Although it is safe and effective in relieving symptoms, recurrence is high. Under appropriate thyroid hormone substitution, most effusions disappear within months. However, the resolution can exceed one year [
3].
The gastrointestinal tract is the primary site of infection with nontyphoidal salmonella. Secondary bacteraemia is uncommon and estimated to occur in approximately 5% of patients with gastroenteritis. However, subclinical intestinal infections can occur and bacteraemia without clinical evidence of gastrointestinal involvement has been reported. Bacteraemia typically occurs in immunocompromised individuals such as in patients with acquired immunodeficiency syndrome (AIDS) or in patients under therapeutic immunosuppression, but bacteraemia has also been reported in immunocompetent subjects [
4,
5,
6].
Haematogenous infections of the cardiovascular system due to nontyphoidal salmonella are well known, but rare. Pericarditis due to nontyphoidal salmonella in an apparently immunocompetent host is extremely infrequent. We found only two case reports, where pericarditis due to nontyphoidal salmonella was diagnosed in immunocompetent patients [
6,
7]. Presence of pericardial fluid at the time of haematogenous spread might act as a culture medium for salmonella [
4,
5].
Thus, the occurrence of pericardial salmonella enteritidis in our patient might rather be the result of superinfection of preexisting hypothyroidic pericardial effusion after transient bacteraemia during an episode of clinically inapparent gastrointestinal infection than “true” salmonella pericarditis.
Figure 1.
Enlarged cardiac silhouette, pleural effusion on the left side.
Figure 1.
Enlarged cardiac silhouette, pleural effusion on the left side.
Figure 2.
Massive pericardial effusion.
Figure 2.
Massive pericardial effusion.
The treatment of choice in pericardial salmonella infection is drainage of large effusions and antibiotic therapy with fluoroquinolones [
4]. There is no consensus about the duration of antimicrobial therapy in salmonella pericarditis. However, most authors propose a minimum of three weeks [
8]. During follow-up, no stool cultures were obtained. Thus it remains unknown, if the patient was a chronic carrier of salmonella.
Pericardial to serum ratios of glucose and LDH were 0.46 and 0.09, respectively. In contrast to pleural effusion or ascites, where these values would be strongly associated with bacterial infection, there are no biochemical or cell-count parameters, which are useful in differentiating individual causes of pericardial effusions [
9]. However, in unclear situations, not only therapeutic drainage but also diagnostic analysis of the pleural fluid is crucial.
Learning points
Large pericardial effusion can evolve in hypothyroidism, and may not be noticed by clinicians due to its insidious onset and slow fluid accumulation, thus evading cardiac tamponade.
Infective pericarditis due to nontyphoidal salmonella in immunocompetent subjects is extremely rare. Presence of pericardial fluid at the time of bacteraemia might act as a culture medium for haematogenously spread salmonella.
In unclear situations, especially subacute, chronic or non-resolving pericardial effusion, not only therapeutic drainage but also diagnostic analysis of the pleural fluid is crucial.