Summary
Catheter ablation of persistent atrial fibrillation (AF) is challenging and needs a combination of different techniques targeting a wider substrate to be effective. The so-called stepwise approach progressively targets structures potentially contributing to initiation and maintenance of AF. The endpoints of the procedure consist of pulmonary vein isolation, organisation and slowing of left and right atrial electrograms and linear block in the left atrial (LA) roof and mitral isthmus lines. This strategy results both in high rate of AF termination (85%) and an unprecedented clinical outcome.
Electrogram-based ablation is crucial for the procedural outcome. While continuous and fractionated electrograms are targeted during complex atrial activity, more discrete sites like temporal activation gradient, rapid or centrifugal activity are ablated when atrial activity is organised allowing discrete mapping.
AF cycle length (CL) measured in both appendages has been demonstrated to be the strongest independent predictor of procedural AF termination in AF lasting <5 years. Baseline AFCL <140 ms suggests AF termination of less than 70% while >140 ms predicts AF termination of more than 89%. AFCL also allows monitoring impact of each step of catheter ablation. LA ablation is usually associated with prolongation of both LA appendage (LAA) CL and right atrial appendage (RAA) CL, while prolongation of LAA CL without concomitant significant prolongation in RA suggests the presence of drivers in RA, requiring additional RA ablation.
Because AF recurrence after the first procedure occurred in only 5% of the cases when AF terminated during the procedure vs 45% if AF persisted, AF termination represents an incontrovertible endpoint of the ablation process.
The mode of termination of persistent AF is conversion to multiples atrial tachycardia (AT) in the vast majority of cases. Mapping and ablation of those have become indispensable steps in the ablation process. Predominant ATs are localised re-entries requiring mapping of local activity spanning most of the AT cycle length in the area of earliest activity.
In conclusion, catheter ablation of persistent AF requires isolation of all PV electrogram based ablation and linear lesions in the majority of patients. AF CL is an important guide for monitoring progress of ablation and reliably predicts the procedural outcome. AF termination by ablation is associated with excellent outcome. Mapping and ablation of subsequent atrial tachycardias are an integral part of the AF ablation process and its success often makes the difference between cure and persistent illness.
Introduction
Atrial fibrillation (AF) is the most frequent arrhythmia in human. Its prevalence of 2% in the general population reaches 5.9% for a population of more than 65 years [1]. Pharmacological therapies have a limited efficacy for the treatment of this arrhythmia, and this particularly in the long run. On the other hand, catheter ablation has been proven to have high success rates in the short and middle runs, and this technique is now included in the guidelines as a “reasonable alternative to pharmacological therapy to prevent recurrent AF in symptomatic patients” (class IIa recommandation, level of evidence C) [2]. The vast majority of paroxysmal AF patients can be successfully treated by electrical isolation of pulmonary veins (PV) [3,4,5,6], but PV isolation alone with or without including the posterior LA is rarely effective in treating patients with long lasting AF [7,8,9,10]. Inefficacy of this approach for long lasting AF suggests substrate modification and presence of drivers in other structures like LA tissue, inferior LA/CS interface and structures outside LA (right atrium, SVC). Subsequent ablation strategies like targeting complex fractionated atrial electrograms (CFAE) [11], areas of short cycle length activity [12], sites of dominant frequency [13,14], performing LA linear lesions to interrupt re-entrant wavelets and reduce LA mass [10,15,16], and cardiac autonomic denervation [17,18] have been reported in the context of ablation for long lasting AF. Even if each of these techniques has only a limited efficacy for ablation of persistent AF, the combination of all in a stepwise approach has resulted in an unprecedented level of acute termination of long lasting AF by catheter ablation.
The stepwise approach for persistent atrial fibrillation
The so-called stepwise approach progressively targets structures potentially contributing to initiation and maintenance of AF: PV, LA tissue, linear ablation of LA roof and mitral isthmus, and right atrium. Each region is ablated following a sequential approach, and the impact of ablation is assessed by measurement of AF cycle length (AF CL) in both appendages. Each step is accompanied by an increase in AF CL until conversion of AF directly to sinus rhythm or more often to multiple atrial tachycardias (AT), which are systematically ablated. The specific sequence of ablation doesn’t seem to play a role in the overall success rate of the ablation. However, given the fact that there is a limited success rate of complete block for linear lesions with a higher rate of complications (particularly for the mitral isthmus line), this step is in general performed as the last one.
Patient selection
Patients with symptomatic persistent AF despite intake of at least one anti-arrhythmic drug, electrical cardioversion, or both are considered for ablation. Patients with heart failure (NYHA II or more) or evidence of left ventricular dysfunction without alternative explanation may represent the group with highest benefit from catheter ablation [19,20,21]. Those with AF related thromboembolism are also considered for the procedure. All antiarrhythmic medications are discontinued at least five half lives prior to the procedure, with the exception of amiodarone which is continued if already prescribed or is prescribed if ECG AF CL is less than 140 ms [22]. Oral anticoagulation with an INR target of 2.5 (between 2 and 3) is prescribed for at least one month before the procedure and transoesophageal echography is performed maximum 48 hours prior to the procedure to exclude intra-atrial thrombus.
Atrial fibrillation cycle length
Baseline measurements of AF CL is systematically performed at the right and left appendages as the average of at least 30 cycle lengths [23], using an automated software (Bard EP) with the annotated electrograms verified to ensure accuracy. Even if atria generally present chaotic activity preventing cycle length measurement, both appendages display discrete and unambiguous electrograms. Interelectrogram intervals of less than 100 ms or continuous electrical activity are manually corrected to count as a single interval [23]. We routinely use the AF CL as surrogate markers of the impact of each step of the ablation process. Prolongation of AF CL with ablation [12,24] demonstrated in previous reports supports the use of AF CL as a monitoring tool.
We have previously shown that the baseline AF CL (prior to any ablation) is significantly longer in patients in whom AF terminates during ablation (156 ± 23 ms, n = 52 vs 130 ± 14 ms, n = 8) [12]. The facility with which AF can be terminated by ablation varies inversely with the baseline AF CL. A baseline AF CL < 140 ms correlated with a 69% termination rate, while a baseline AF CL ≥140 ms was associated with a 89% rate of termination during radiofrequency application in a cohort of 178 consecutive patients undergoing catheter ablation. In our experience, AF generally terminates when the AF CL has prolonged to about 180–200 ms. If RA AF CL remains short despite LA AF CL prolongation after LA ablation, it suggests the presence of critical drivers in the RA, which should also be targeted if AF termination is the desired endpoint. The baseline AF CL can be accurately measured on the 12 lead ECG, with a good correlation to the LA AF CL in more than 90% of the cases [25]. Patients with a short AF CL determined with the 12 lead ECG can be pretreated with amiodarone in order to prolong AF CL at the time of the procedure, and to improve the success rate [22].
Termination
Even if it looks intuitively to be associated with a better outcome, termination of persistent AF during the ablation procedure has not yet been validated as an endpoint by the scientific community given the risk-benefit ratio. However, recent follow-up of our patients strongly supports a favourable outcome following AF termination. From a cohort of 153 patients with permanent AF, after a follow-up of 22 ± 10 months, AF termination during the ablation procedure was associated with a 95.3% success rate (90% without any antiarrhyhthmic drug), while persistent AF requiring electrical or chemical cardioversion at the end of the procedure was associated with a 50% success rate. This success rate was achieved with more than one procedure in 53% of the patients whom AF terminated, and in 71% of patients with persistent AF at the end of the procedure. Furthermore, mode of arrhythmia recurrence differed in relation to AF termination. Indeed, AF recurrence occurred in only 5% of the cases when AF terminated during the procedure vs 45% if AF persisted, while AT recurred in 42% of the former patients vs 26% in the latter.
Thus, AF termination besides representing an immediate procedural gratification is an incontrovertible endpoint and is associated with better clinical outcome.
Sequential approach
- Pulmonary vein isolation
PV isolation is performed as the initial ablation step in all the patients with persistent AF. A circumferential catheter is used to map and guide ablation of each vein. Power is limited to 48 °C with a target tissue temperature of 42 °C. Irrigation rates of between 5 and 60 ml/ min are employed (0.9% saline via Cool Flow, Biosense Webster) and manually titrated by the nurse during radiofrequency energy delivery to achieve the desired power and temperature. The power is limited to 25 W for the posterior part of PVs according the possible presence of the oesophagus whom position is not systematically determined. PV ostia are routinely determined by advancing the catheter inside the vein with downward deflection of the tip, and then dragging back with fluoroscopic monitoring of the drop off. Ablation is performed one centimetre apart from the ostia to avoid risk of vein stenosis. For the anterior aspect of the veins, a power of 25 W is used for the lefts veins since ablation from within the proximal portion of the vein is often required, and 30 to 35 watts are applied for the right veins. Radiofrequency energy is delivered continuously for 30 to 60 seconds at each point and prolonged to 1 to 2 minutes when a visible change in the activation sequence or electrogram morphology is observed. Targets are earliest activity recorded with the circumferential catheter, and electrogram polarity reversal [26]. Veins are isolated individually or as ipsilateral pairs depending on venous anatomy, issues with catheter stability and operator’s preference. If performed “en bloc”, right PV isolation is first performed anatomically using fluoroscopy, and then assessed by circumferential catheter which is moved from one to the other vein to determine gaps in the encircling ostial line.
Left PVs can not be routinely isolated “en bloc”, because of the presence of the left atrial appendage anterior to the veins rending completely unstable the position of the ablation catheter on the narrow rim separating both structures. After the ablation of the posterior wall, energy must be delivered within the first millimetres of the veins to achieve effective disconnection.
For all veins, isolation is assessed by either electrical elimination or dissociation of the PV potentials.
- Electrogram based LA and CS Ablation
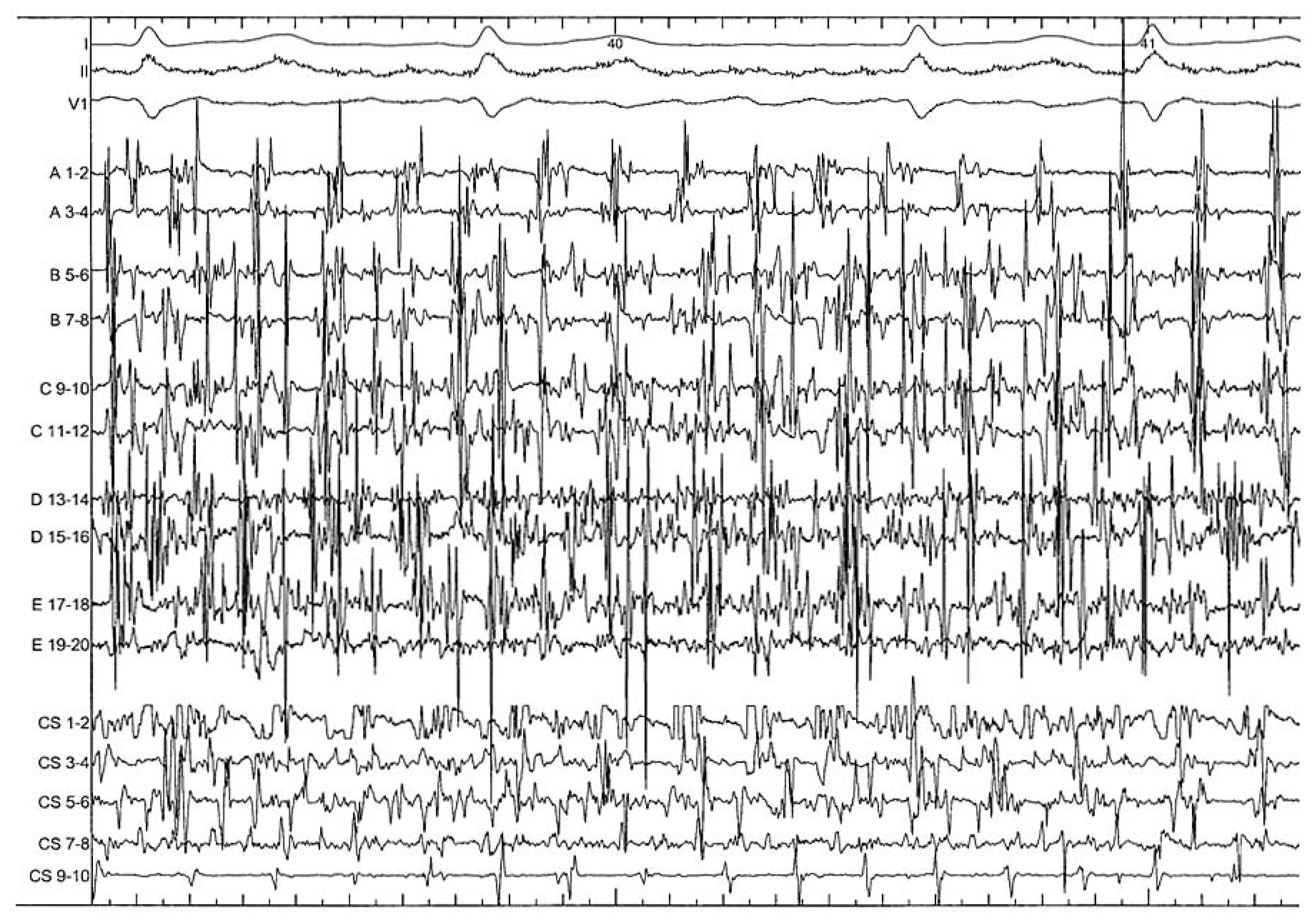
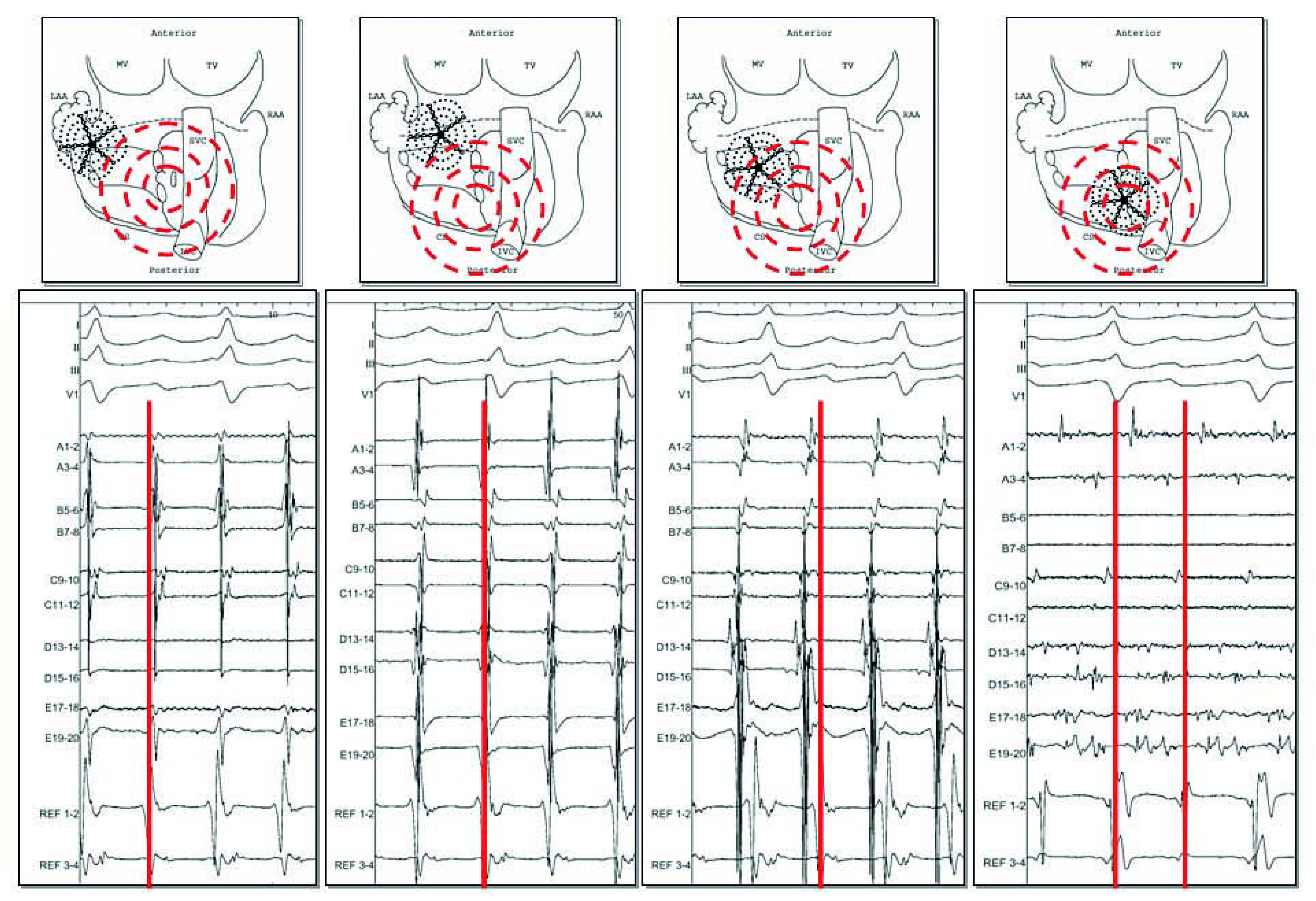
After PV isolation, atrial activity is most of the time chaotic and too complex to allow discrete analysis of the local cycle length or activation. As a first step, only analysis of morphology or frequency content can be realised, and target sites include continuous activity, complex fractionation or rapid activity [12] (Figure 1). Sites displaying potential targets can be found in all parts of the LA, even if LAA and the interface between inferior LA and coronary sinus seem to have the most important impact on the AF CL [12,23,27]. At this step, the endpoint of ablation of the LAA is slowing and organisation of local activity.
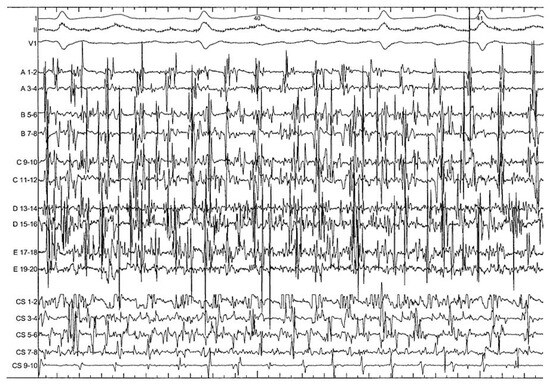
Figure 1.
After PV isolation, high complex pattern of the anterior LA recorded by a 20 poles pentaray catheter (from A to E) and of the coronary sinus (decapolar catheter CS from 1 to 10) in the context of persistent AF. These areas displaying continuous activity have to be organised and slowed before allowing more discrete analysis of the local cycle length and activation.
LAA ablation is performed at the top, anterior and posterior part separating this structure from the PVs, but without fully encircling this structure given its potential role in LA function [28] and the need for anticoagulation in case of complete disconnection. A loop can be performed to achieve stability by advancing the sheath inside the LAA and ablation catheter turning all around the LAA junctions. The posterior wall separating LAA from left veins is a frequent site of complex activity and is systematically checked. A power between 25 and 30 watts is used depending on the contact and position of the ablation catheter to avoid steam pops and subsequent perforation [29].
Inferior LA ablation is performed by looping the ablation catheter in the LA, allowing a very stable position along the posterior mitral annulus. The catheter is dragged along the CS from septal to lateral inferior LA (from 7 o’clock to 4 o’clock in the left anterior oblique projection) aiming disconnection of inferior LA/CS interface. Artifacts can generally be observed within the CS on the electrodes facing the ablation catheter during the ablation. The endpoint is elimination of local electrograms, and this is often accompanied by some organisation of activity inside the CS [27].
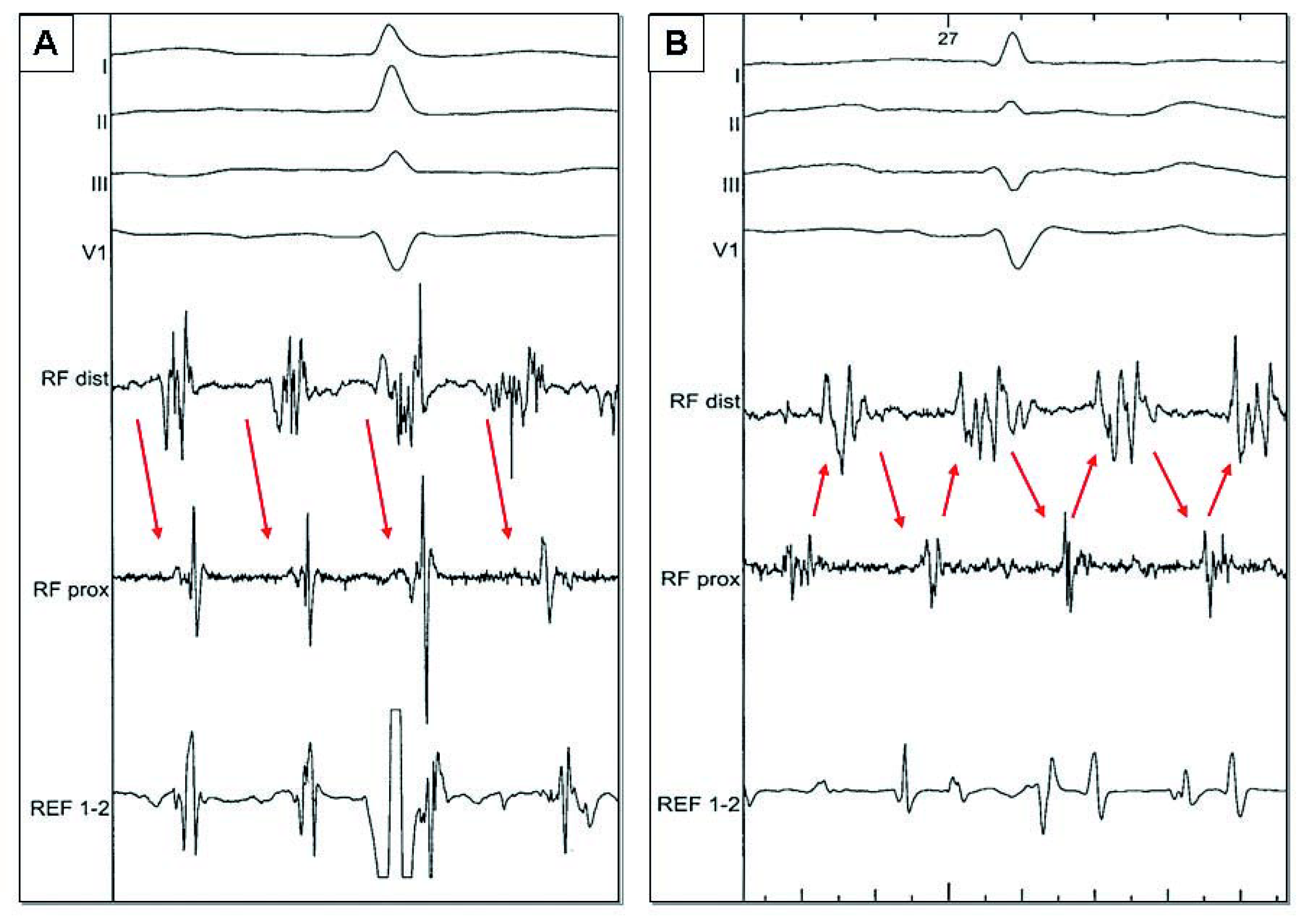
Ablation from within the CS may be required to eliminate electrophysiological targets which may be potential drivers of AF [27]. Power is limited to 25 W and the irrigation rate adjusted manually to achieve the desired power setting, with the highest irrigation flow rates required for ablation in the distal CS (up to 60 ml/min). Ablation is started distally CS (approximately 4 o’clock in the LAO projection) and pursued along the CS up to the ostium with the tip deflected towards the LA. This may help to minimise the risk of circumflex coronary artery injury [30]. Ablation is performed point by point or continuously depending on the extent of complex local activities. The endpoint of ablation within the CS is local organisation and slowing of electrical activities. Complete disconnection of this structure is not routinely attempted in order to minimise the complication rate, and based on a prior study which demonstrated little additional benefit with total disconnection of the CS [12]. After having “cleaned” the most complex areas, a progressive prolongation of AF CL is observed and local as well as global atrial activities become more organised. A more subtle electrogram based approach can then be used to detect most favourable sites which may represent atrial sources (Figure 2). Targets include (1.) areas of temporal gradients between activities recorded on the proximal and distal bipoles or between adjacent areas (potentially representing a small circuit), (2.) discrete sites displaying centrifugal activation (with a distal to proximal pattern becoming proximal to distal after slightly advancing the mapping catheter), (3.) evidence of electrical activity originating from a dead end area (with a distal to proximal pattern for example from the bottom of the appendage), and area of local short cycle length (faster than LA AF CL) [31,32].
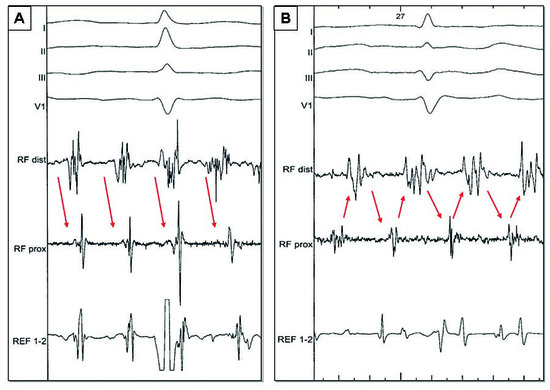
Figure 2.
During electrogram based approach, 2 examples of sites which can potentially represent sources. A A distal to proximal activity (arrows) can be targeted in case of dead end area (left appendage) or if activation become proximal to distal by advancing slightly the mapping catheter (centrifugal activation). B Temporal gradient (arrows) between activities recorded on proximal and distal bipoles (potentially representing a small circuit).
In our experience, termination of AF occurred in 73% of cases during electrogram based ablation after PV isolation [12].
- Right atrium and SVC
Because of its easier access, RA was historically the first target for AF ablation, but with modest success even with extensive ablation. However, in 15% of the patients in whom we fail to restore sinus rhythm in the LA and associated structures [12], some data suggest the presence of perpetuators of AF in the RA or SVC [14,33,34,35,36]. This situation is expected when during LA ablation only a relative prolongation of the CL occurs in the RA [37]: a shorter AF CL in RA appendage compared to LAA by more than 15–20 ms would suggest that RA may harbor the perpetuating influence for ongoing AF (Figure 3). Similarly pauses in activity in LA electrograms recordings not followed by pauses in the RAA would suggest RA as the culprit driver. As was the case with the LA, the targets for ablation are regions displaying complex electrical activity and after slowing and organisation of the chamber potential sources (Figure 4). Preferential areas are RAA, which can be mapped and ablated with the circumferential ablation catheter to determine the fastest and earliest sites of activation, intercaval region and CS ostium. SVC is isolated only when there is evidence of arrhythmogenicity in the vessel (activation from the vein or local rapid activity). Care must be taken to avoid phrenic nerve and sinus node injury during ablation in this region [38]. Finally, cavotricuspid isthmus (CTI) ablation is performed in all patients.
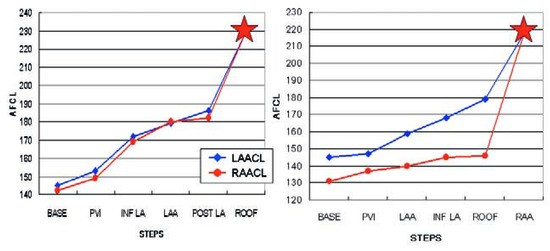
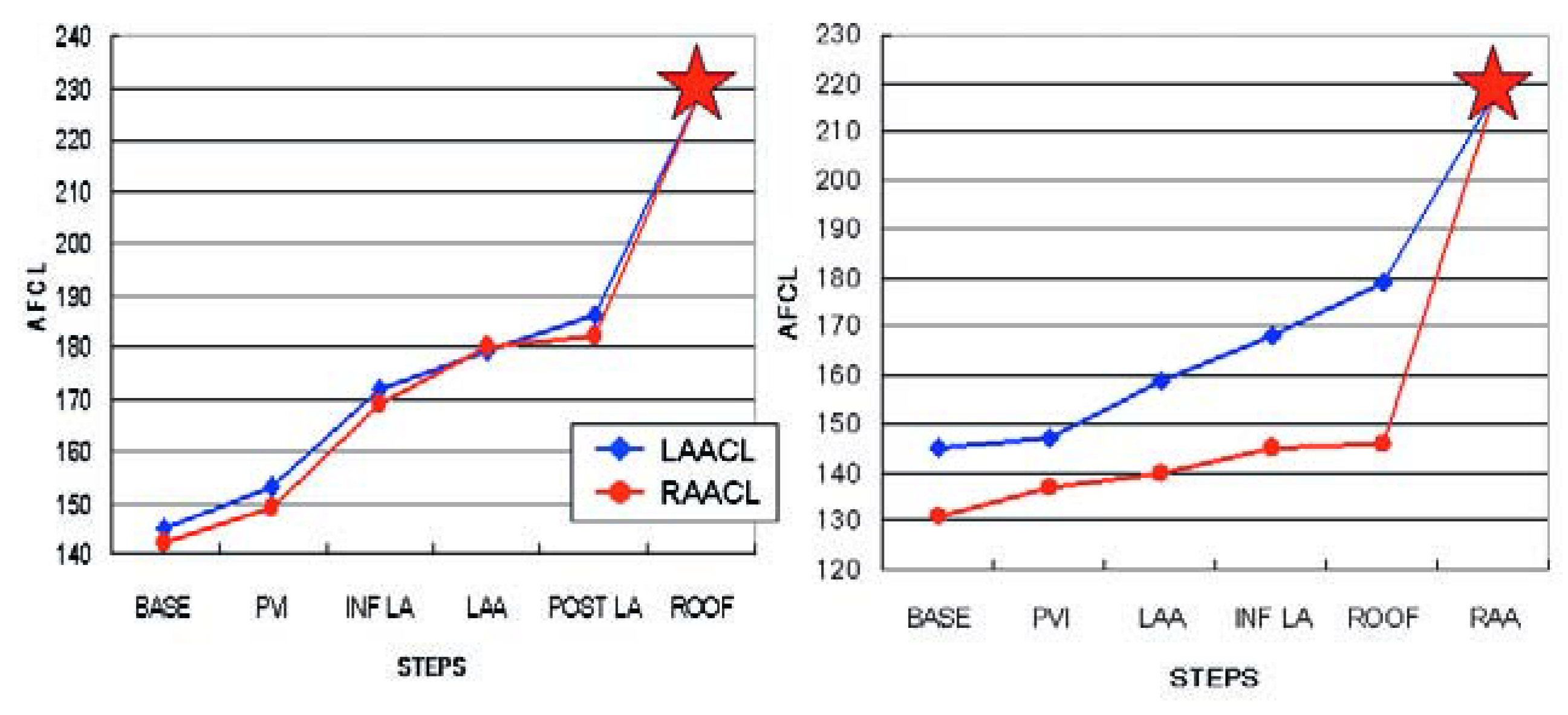
Figure 3.
A Progressive prolongation of both left (LAA) and right (RAA) atrial appendages cycle lengths (CL), until conversion to atrial tachycardia (red star) which occurs at AF CL of 183 (LAA CL) and 181 (RAA CL). B Lesser prolongation of the RAA CL during LA ablation, indicating driving activities in the RA. Ablation of the RAA which converted AF to AT (red star).
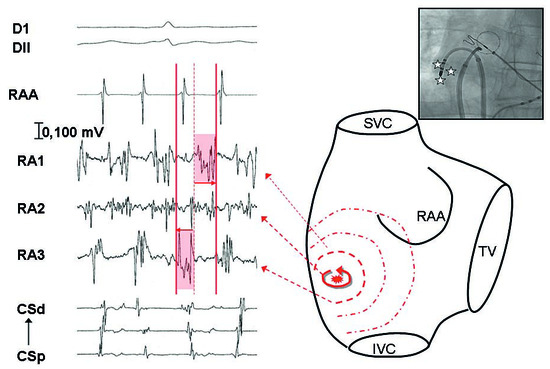
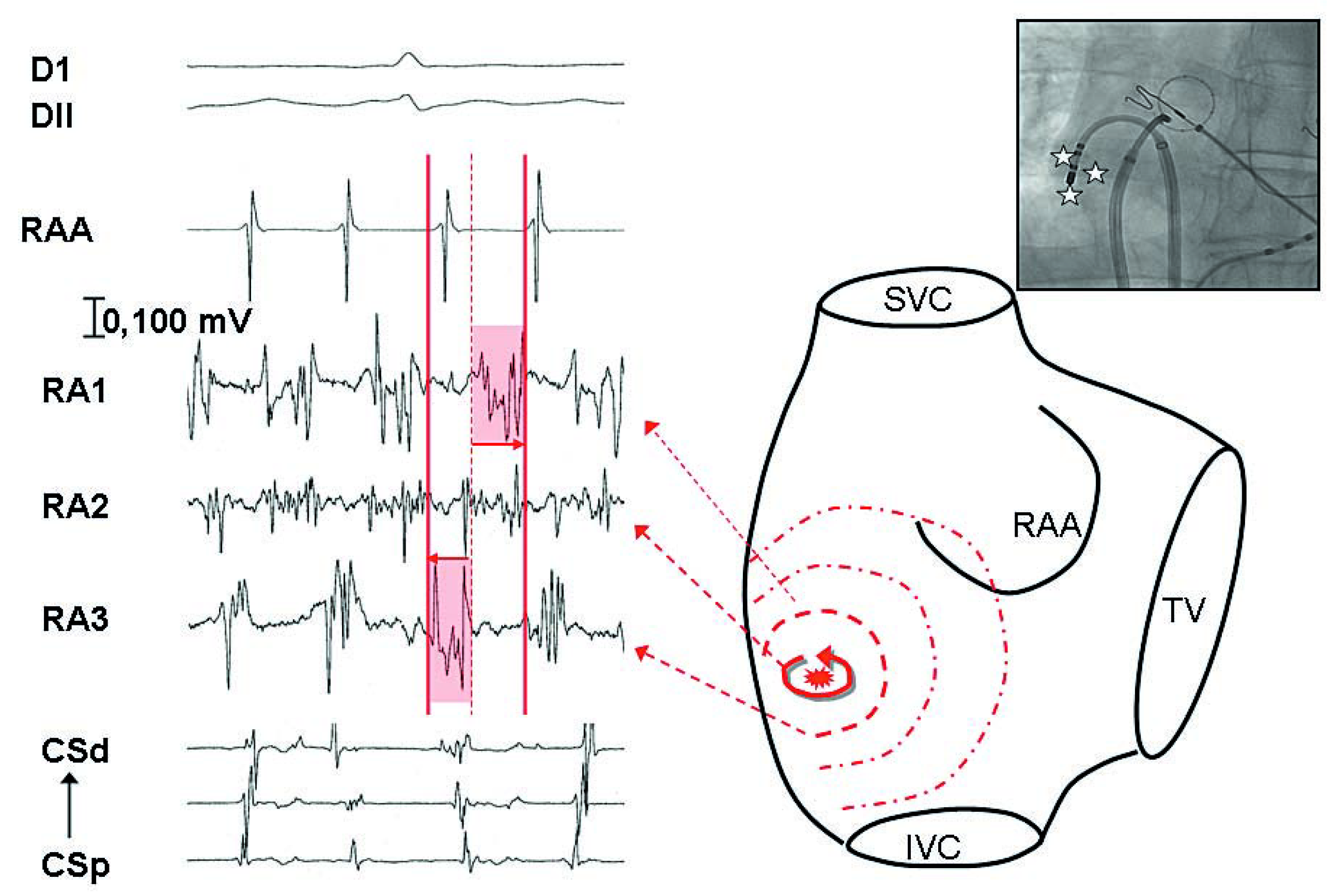
Figure 4.
Recording of the mapping catheter (RA 1 to 3) positioned in the lateral right atrium, of the right appendage with the circumferential catheter (RAA), and of the coronary sinus (CS). By moving mapping catheter in a small area, recording of temporal gradient between 2 adjacent points (RA1 and RA3) possibly representing a small source. In between, recording of a continuous activity which was targeted with subsequent restoration of sinus rhythm.
Linear Ablation
LA Roof line
Because linear ablation of the roof is performed by connecting margins of both superior PVs, its length depends on extension of PV encircling lesions. It can be achieved in 96% of the cases during 12 ± 6 minutes of RF delivery [39]. The line is performed at the most cranial part of the LA to minimise the risk of oesophageal injury. Three different approaches can be applied: perpendicular to the roof (with a reduced power to 25 watts to minimise the likelihood of steam pop and perforation [29]), parallel to the roof by looping the catheter in the LA or by steering the sheath in one direction and the catheter in another [39]. In this less aggressive position, a power up to 30 watts can be used and higher irrigation rates may be required due to occlusion of the irrigation holes with this orientation. During AF, the endpoint is the elimination of local electrograms. Following ablation, a complete line of conduction block is confirmed in sinus rhythm using following manoeuvres [39]: in the presence of a complete roof line, during LAA pacing (using the CS catheter positioned through the transseptal puncture), activation of the posterior LA becomes inferior to superior compared to the opposite activation sequence before ablation. Furthermore, the posterior LA is activated from left to right, if the mitral isthmus is not blocked and from right to left, if blocked (the conduction progressing across the anterior LA, down the interatrial septum to the posterior LA from the right).
Left mitral isthmus line
Mitral isthmus line is the most challenging step in the process of persistent AF ablation. Block can be achieve only in a limited percentage of cases (± 90%), and failures can have proarrhythmic consequences [40,41]. Furthermore, there is a risk of periprocedural tamponade of 1–2% [15]. In 70% of the cases, it is necessary to extend the ablation within the distal coronary sinus to achieve block. As a consequence, in patient in whom this structure can not be catheterised, this step should be avoided if possible. However, its ablation has been shown to increase the success rate of catheter ablation for drug-refractory long lasting AF [15]. The ablation catheter is positioned with an angle from 90° to 180° at the ventricular side of mitral isthmus where the atrial to ventriculogram ratio is 1:1 or 2:1. Radiofrequency is applied using power from 30 to 35 watts, and irrigation rates up to 60 ml/min. Ablation is continued by turning clockwise both sheath and ablation catheter to reach the left inferior PV. Careful monitoring of the stability of the catheter is necessary to avoid inadvertent displacement to the left inferior PV or LAA, to prevent vein stenosis or perforation. The completeness of block is monitored with the CS catheter bridging both parts of the line. It is often necessary to extend the line to the base of the LAA and inside the coronary sinus (with a limited power at 25 watts) in order to achieve isthmus block.
A complete line is confirmed in sinus rhythm with the following criteria: local corridor of equidistant double potentials, inversion of CS activation sequence during pacing from LAA and confirmation of bidirectional block by differential pacing from proximal and distal CS pacing to the recording catheter placed in the LAA.
Mapping and ablation of resultant atrial tachycardia
Because of electrogram based ablation creating the substrate for subsequent re-entries, mapping and ablation of atrial tachycardias are indispensable steps in the AF ablation process.
Restoration to sinus rhythm requires ablation of one or more ATs in 87% of patients (mean: 2.6 ± 2 AT per patient; range 2–6 AT per patient) [12], including 50% of each macroreentrant and focal tachycardia. As a first step, we always confirm PV isolation. In our experience, AT variability of more than 15% strongly suggests macroreentry. Among them, perimitral flutter is the most common (30% of the patients), before reentry around veins (roof dependant) (12%) and peritricuspid circuit (8%). Rapid anterior and posterior LA mapping can suggest the diagnosis. For perimitral flutter both anterior and posterior activation wavefronts are ascending, with consistent activation around the mitral annulus covering all the cycle. For a roof dependant circuit one activation wavefront is ascending and the other one is descending with all the cycle covered around the right or left veins. For a peri tricuspid circuit, both anterior and posterior LA activation wavefronts are ascending and activated from septal to lateral, and all the cycle is covered throughout the tricuspid annulus. Entrainment manoeuvres are currently used to confirm the diagnosis. In absence of macroreentry, we track a discrete source with centrifugal activation. In our experience, about 70% of these focal tachycardias in the context of prior AF ablation are localised reentry [42] (Figure 5). Those, described by Sanders and al. with high density mapping [42], display centrifugal activation with the entire tachycardia cycle length within a localised region (<2 cm) at the AT origin. Using conventional mapping, the global strategy is to determine areas of earliest activity. Entrainment manoeuvres are routinely used with the goal to determine a post pacing interval lower than 20–30 ms [43]. Localised reentry are then tracked, by looking for fractionated electrogram covering more than 75% of the cycle length in a small area of less than 2 cm [42]. In case of focal point tachycardia, the earliest activity is targeted. The more frequent locations of focal AT are the base of the LAA, the septum and the PVs, but they can occur everywhere in both atria.
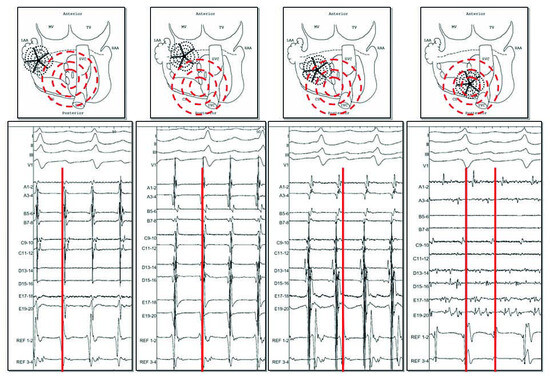
Figure 5.
Atrial tachycardia occurring in the context of prior long lasting AF ablation. With the 20 poles pentaray catheter, recording of an organised activity. By moving this catheter from lateral LA to septal LA (from A to D), activity becomes earlier compared to a reference in the coronary sinus (red line). In the earliest area of less than 2 cm, recording of local activity spanning all the cycle length (D, between 2 lines) and diagnosed as a localised reentry. Ablation at this region restored sinus rhythm.
With this algorithm based approach, ATs are diagnosed and ablated in 95% of the cases.
Conclusions
Persistent AF requires extensive radiofrequency ablation to be cured, associating pulmonary vein isolation, electrogram based ablation and linear lesions for most of the patients. AF cycle length is the strongest predictor of procedural outcome. It is also the only reliable monitoring tool to guide ablation during the procedure, in particular to suggest right atrial perpetuators. AF termination by ablation is highly predictive of an outstanding clinical outcome. Mapping and ablation of subsequent atrial tachycardias are an integral part of the AF ablation process and its success often makes the difference between cure and persistent illness.
Funding
Sébastien Knecht is supported by the Belgian “Funds for cardiac surgery” and Mark O’Neill is supported by the British Heart Foundation.
References
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The Framingham heart study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef]
- Fuster, V.; Ryden, L.E.; Cannom, D.S.; et al. ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/ American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for practice guidelines (Writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006, 114, e257–e354. [Google Scholar]
- Hocini, M.; Sanders, P.; Jais, P.; et al. Techniques for curative treatment of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2004, 15, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Oral, H.; Chugh, A.; Good, E.; et al. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation 2006, 113, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Marrouche, N.F.; Natale, A. Pulmonary vein antrum isolation: Intracardiac echocardiography-guided technique. J. Cardiovasc. Electrophysiol. 2004, 15, 1335–1340. [Google Scholar] [CrossRef]
- Pappone, C.; Santinelli, V. Atrial fibrillation ablation: State of the art. Am. J. Cardiol. 2005, 96 (12, Suppl. 1), 59–64. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.; Hocini, M.; Jais, P.; et al. Complete isolation of the pulmonary veins and posterior left atrium in chronic atrial fibrillation. Long-term clinical outcome. Eur. Heart J. 2007, 28, 1862–1871. [Google Scholar] [CrossRef]
- Oral, H.; Knight, B.P.; Tada, H.; et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002, 105, 1077–1081. [Google Scholar] [CrossRef]
- Kanagaratnam, L.; Tomassoni, G.; Schweikert, R.; et al. Empirical pulmonary vein isolation in patients with chronic atrial fibrillation using a three-dimensional nonfluoroscopic mapping system: Long-term follow-up. PACE. 2001, 24, 1774–1779. [Google Scholar] [CrossRef]
- Willems, S.; Klemm, H.; Rostock, T.; et al. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: A prospective randomized comparison. Eur. Heart J. 2006, 27, 2871–2878. [Google Scholar] [CrossRef]
- Nademanee, K.; McKenzie, J.; Kosar, E.; et al. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004, 43, 2044–2053. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Sanders, P.; Hocini, M.; et al. Catheter ablation of long-lasting persistent atrial fibrillation: Critical structures for termination. J. Cardiovasc. Electrophysiol. 2005, 16, 1125–1137. [Google Scholar] [CrossRef]
- Jalife, J.; Berenfeld, O.; Mansour, M. Mother rotors and fibrillatory conduction: A mechanism of atrial fibrillation. Cardiovasc. Res. 2002, 54, 204–216. [Google Scholar] [CrossRef]
- Sanders, P.; Berenfeld, O.; Hocini, M.; et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 2005, 112, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Jais, P.; Hocini, M.; Hsu, L.F.; et al. Technique and results of linear ablation at the mitral isthmus. Circulation 2004, 110, 2996–3002. [Google Scholar] [CrossRef]
- Kottkamp, H.; Hindricks, G.; Autschbach Ru et, a.l. Specific linear left atrial lesions in atrial fibrillation: Intraoperative radiofrequency ablation using minimally invasive surgical techniques. J. Am. Coll. Cardiol. 2002, 40, 475–480. [Google Scholar] [CrossRef]
- Pappone, C.; Santinelli, V.; Manguso, F.; et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004, 109, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Scherlag, B.J.; Nakagawa, H.; Jackman, W.M.; et al. Electrical stimulation to identify neural elements on the heart: Their role in atrial fibrillation. J. Interv. Card. Electrophysiol. 2005, 13, 37–42. [Google Scholar] [CrossRef]
- Hsu, L.F.; Jais, P.; Sanders, P.; et al. Catheter ablation for atrial fibrillation in congestive heart failure. N. Engl. J. Med. 2004, 351, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.D.; Sanders, P.; Takahashi, Y.; et al. Treatment of chronic atrial fibrillation by catheter ablation in patients with heart failure. PACE. 2006, 29, S8–S9. [Google Scholar]
- Chen, M.S.; Marrouche, N.F.; Khaykin, Y.; et al. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J. Am. Coll. Cardiol. 2004, 43, 1004–1009. [Google Scholar] [CrossRef]
- Sanders, P.; Sacher, F.; Jais, P.; et al. P4–44: Restoration of atrial mechanical function after catheter ablation of AF. impact of the mode of termination and arrhythmia duration. Heart Rhythm. 2006, 3 (5, Suppl. 1), S232–S233. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Sanders, P.; Hocini, M.; et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation 2004, 109, 3007–3013. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Hocini, M.; Sanders, P.; et al. Catheter ablation of long-lasting persistent atrial fibrillation: Clinical outcome and mechanisms of subsequent arrhythmias. J. Cardiovasc. Electrophysiol. 2005, 16, 1138–1147. [Google Scholar] [CrossRef]
- Matsuo, S.; Jais Pierre Hocini, M.; et al. Twelve lead ECG is a reliable estimate of atrial fibrillation rate in patients with chronic AF. Heart Rhythm. 2007, 4 (Suppl. 5), PO3–103. [Google Scholar]
- Yamane, T.; Shah, D.C.; Jais, P.; et al. Electrogram polarity reversal as an additional indicator of breakthroughs from the left atrium to the pulmonary veins. J. Am. Coll. Cardiol. 2002, 39, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Hocini, M.; Takahashi, Y.; et al. Impact of catheter ablation of the coronary sinus on paroxysmal or persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007, 18, 378–386. [Google Scholar] [CrossRef]
- Kamohara, K.; Popovic’, Z.B.; Daimon, M.; et al. Impact of left atrial appendage exclusion on left atrial function. J. Thorac. Cardiovasc. Surg. 2007, 133, 174–181. [Google Scholar] [CrossRef]
- Nakagawa, H.; Wittkampf, F.H.M.; Yamanashi, W.S.; et al. Inverse relationship between electrode size and lesion size during radiofrequency ablation with active electrode cooling. Circulation 1998, 98, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Jais, P.; Hocini, M.; et al. Acute occlusion of the left circumflex coronary artery during mitral isthmus linear ablation. J. Cardiovasc. Electrophysiol. 2005, 16, 1104–1107. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Hocini, M.; Sanders, P.; et al. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation 2006, 113, 616–625. [Google Scholar] [CrossRef]
- Jais, P.; O’Neill, M.D.; Takahashi, Y.; et al. Stepwise catheter ablation of chronic atrial fibrillation: Importance of discrete anatomic sites for termination. J. Cardiovasc. Electrophysiol. 2006, 17, S28–S36. [Google Scholar] [CrossRef]
- Tsai, C.F.; Tai, C.T.; Hsieh, M.H.; et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: Electrophysiological characteristics and results of radiofrequency ablation. Circulation 2000, 102, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Elayi, C.S.; Fahmy, T.S.; Wazni, O.M.; Patel, D.; Saliba, W.; Natale, A. Left superior vena cava isolation in patients undergoing pulmonary vein antrum isolation: Impact on atrial fibrillation recurrence. Heart Rhythm. 2006, 3, 1019–1023. [Google Scholar] [CrossRef]
- Saksena, S.; Skadsberg, N.D.; Rao, H.B.; Filipecki, A. Biatrial and three-dimensional mapping of spontaneous atrial arrhythmias in patients with refractory atrial fibrillation. J. Cardiovasc. Electrophysiol. 2005, 16, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Ishii, Y.; Miyagi, Y.; Ohmori, H.; Sakamoto Si Tanaka, S. Concurrent multiple left atrial focal activations with fibrillatory conduction and right atrial focal or reentrant activation as the mechanism in atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2004, 127, 770–778. [Google Scholar] [CrossRef]
- O’Neill, M.D.; Jonsson, A.; Hocini, M.; et al. P5–78: Sigmoidal relationship between extent of left atrial ablation and termination of chronic atrial fibrillation. Heart Rhythm. 2006, 3 (5, Suppl. 1), S286. [Google Scholar] [CrossRef]
- Sacher, F.; Monahan, K.H.; Thomas, S.P.; et al. Phrenic nerve injury after atrial fibrillation catheter ablation: Characterization and outcome in a multicenter study. J. Am. Coll. Cardiol. 2006, 47, 2498–2503. [Google Scholar] [CrossRef]
- Hocini, M.; Jais, P.; Sanders, P.; et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: A prospective randomized study. Circulation 2005, 112, 3688–3696. [Google Scholar] [CrossRef]
- Chugh, A.; Oral, H.; Lemola, K.; et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005, 2, 464–471. [Google Scholar] [CrossRef]
- Gerstenfeld, E.P.; Callans, D.J.; Dixit, S.; et al. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation 2004, 110, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.; Hocini, M.; Jais, P.; et al. Characterization of focal atrial tachycardia using high-density mapping. J. Am. Coll. Cardiol. 2005, 46, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, U.; Skanes, A.C.; Gula, L.J.; et al. A novel pacing maneuver to localize focal atrial tachycardia. J. Cardiovasc. Electrophysiol. 2007, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
© 2008 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.