Mechanism of Glucose Water as a Neural Injection: A Perspective on Neuroinflammation
Abstract
:1. Introduction
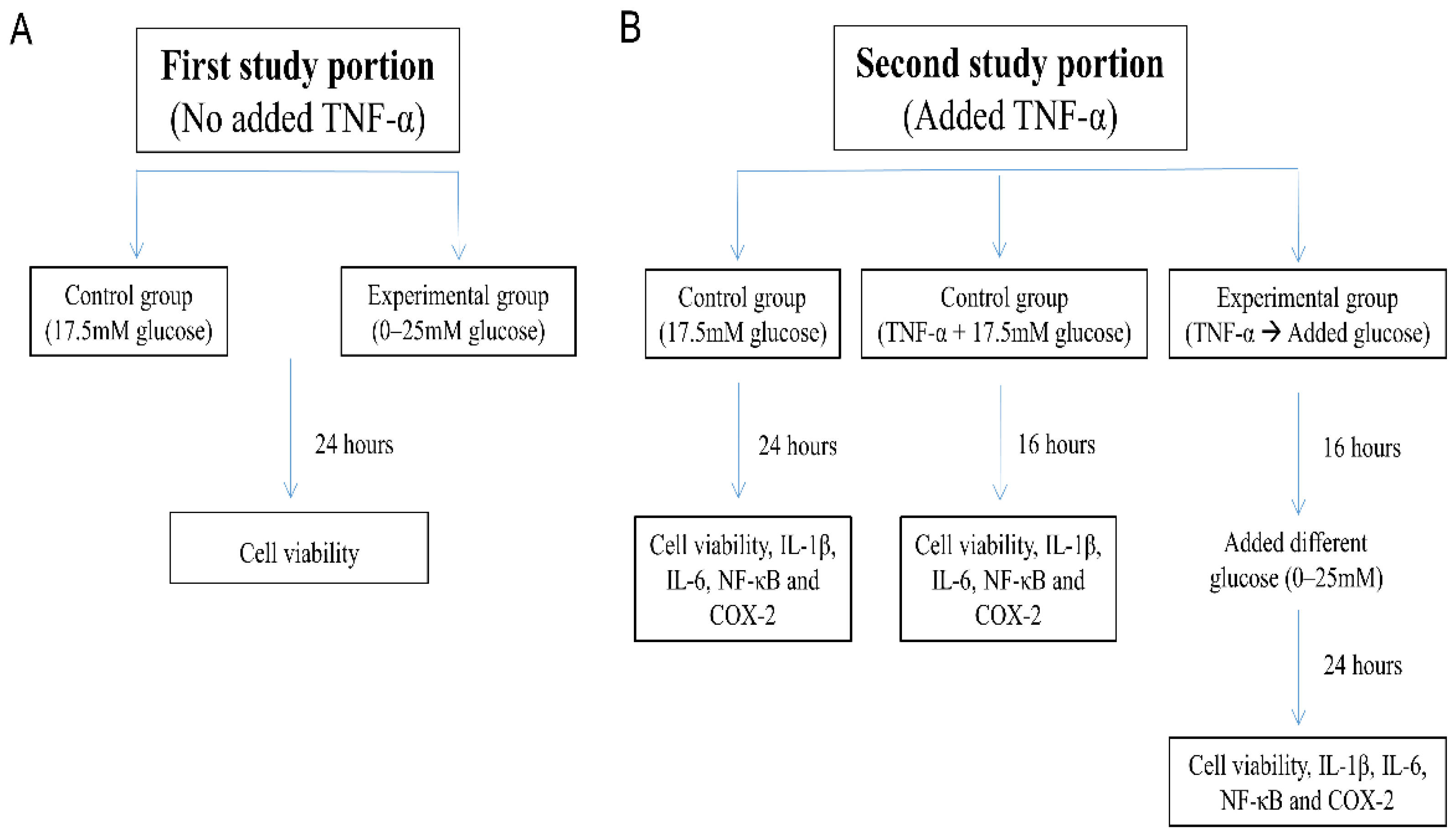
2. Materials and Methods
2.1. Cell Culture
- “17.5 mM glucose control”: SH-SY5Y cells were maintained for 24 h in DMEM/F12 Hams with 17.5 mM glucose;
- “TNF-α + 17.5 mM glucose control”: SH-SY5Y cells exposed to 10 ng/mL TNF-α for 16 h in DMEM/F12 Hams with 17.5 mM glucose; and
- “TNF-α → Added glucose groups”: Washed and harvested SH-SY5Y cells were first pretreated with TNF-α (10 ng/mL) in 1% FBS DMEM/F12 Hams without glucose for 16 h [34], followed by incubation for an additional 24 h with glucose added to the culture medium at 0–25 mM concentration.
2.2. Cell Viability Assay
2.3. ELISA
2.4. Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
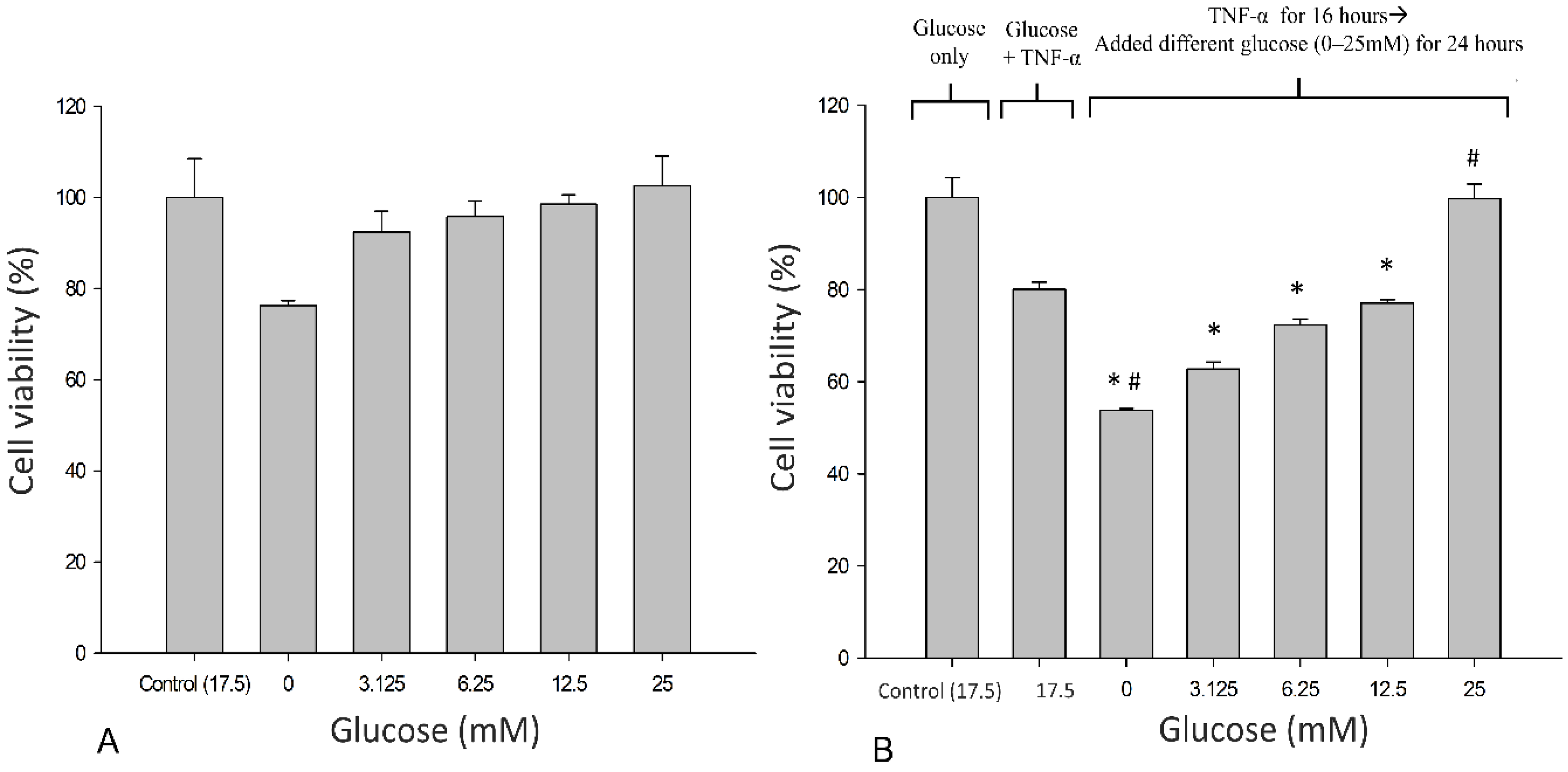
3.1. Effects of Glucose on the Metabolic Activity of SH-SY5Y Cells
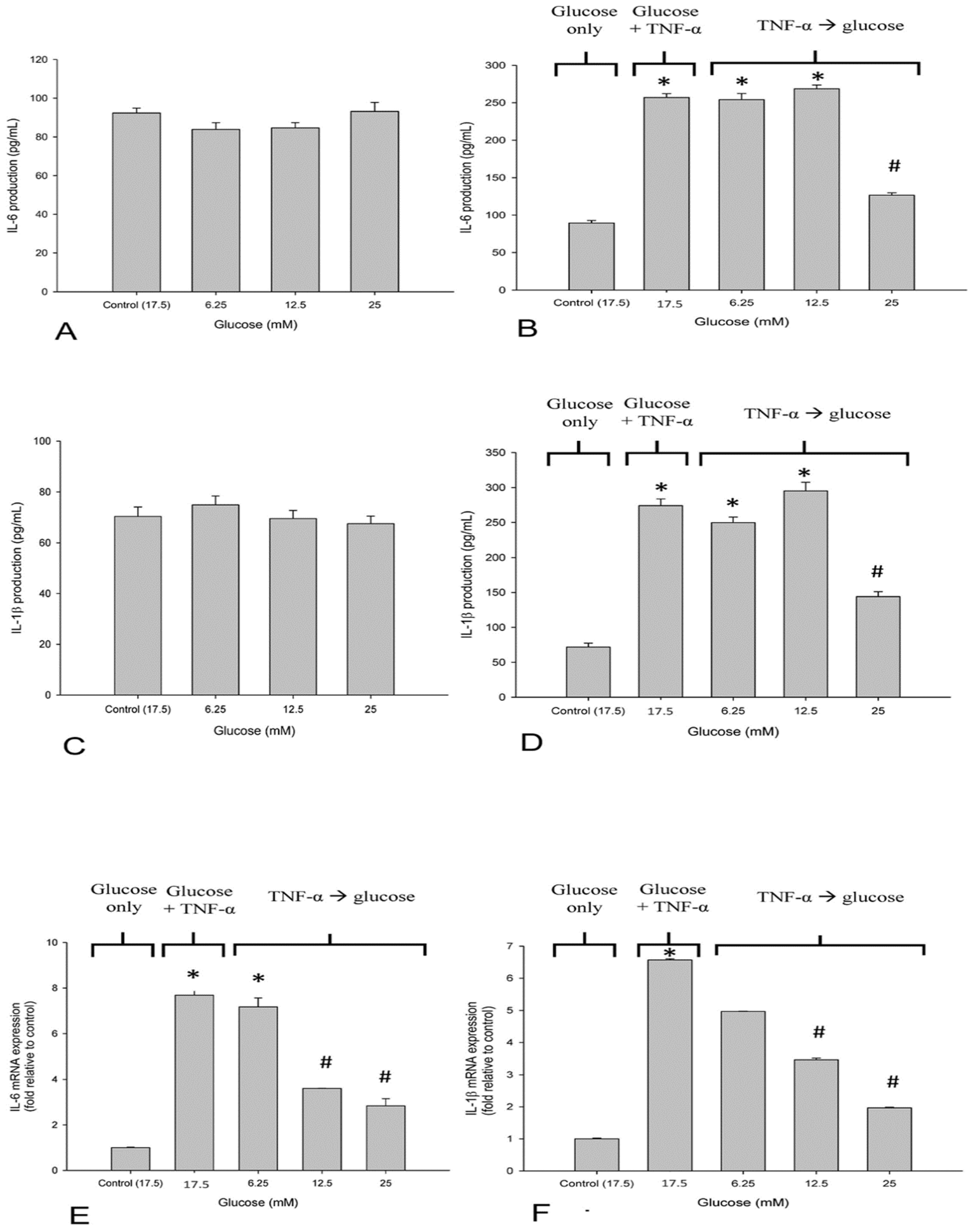
3.2. High Glucose Reduced TNF-α-Induced IL-6 and IL-1β-Production in SH-SY5Y Cells
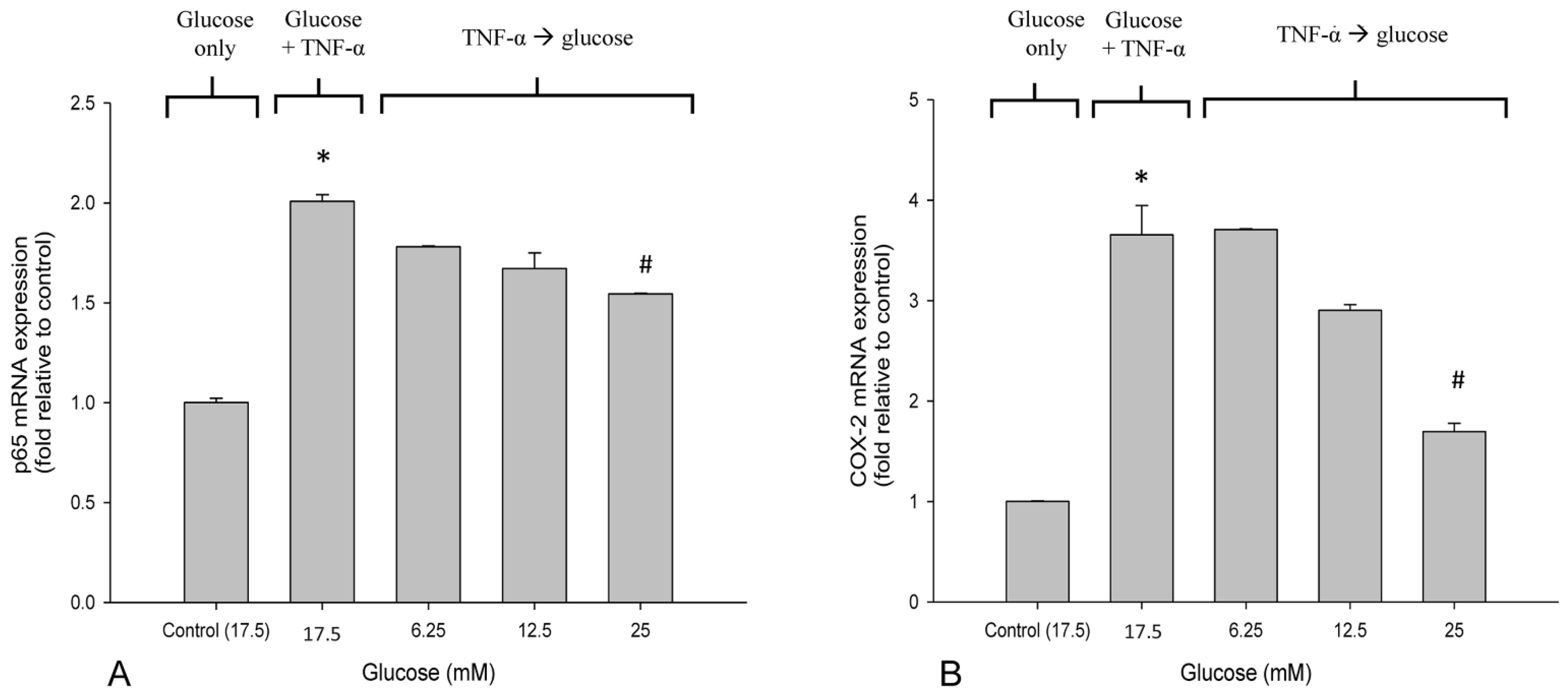
3.3. High Glucose Reduced the Elevated mRNA Expressions of NF-κB and COX-2 in TNF-α-Treated SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, K.V.; Wu, W.T.; Ozcakar, L. Ultrasound imaging and guidance in peripheral nerve entrapment: Hydrodissection highlighted. Pain Manag. 2020, 10, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.B.; Fundaun, J.; Tampin, B. Entrapment neuropathies: A contemporary approach to pathophysiology, clinical assessment, and management. Pain Rep. 2020, 5, e829. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.J.; Singh, N.P.; Robin, J.X.; Sharma, S. Compression Neuropathies of the Upper Extremity: A Review. Surgeries 2021, 2, 320–334. [Google Scholar] [CrossRef]
- van Rijn, R.M.; Huisstede, B.M.; Koes, B.W.; Burdorf, A. Associations between work-related factors and specific disorders at the elbow: A systematic literature review. Rheumatology 2009, 48, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyftogt, J. Subcutaneous prolotherapy for Achilles tendinopathy: The best solution? Australas. Musculoskelet. Med. 2007, 12, 107. [Google Scholar]
- Lam, K.H.S.; Hung, C.Y.; Chiang, Y.P.; Onishi, K.; Su, D.C.J.; Clark, T.B.; Reeves, K.D. Ultrasound-Guided Nerve Hydrodissection for Pain Management: Rationale, Methods, Current Literature, and Theoretical Mechanisms. J. Pain Res. 2020, 13, 1957–1968. [Google Scholar] [CrossRef]
- Lam, K.H.S.; Hung, C.Y.; Chiang, Y.P.; Onishi, K.; Su, D.C.J.; Clark, T.B.; Reeves, K.D. Practical Considerations for Ultrasound-Guided Hydrodissection in Pronator Teres Syndrome. Pain Med. 2022, 23, 221–223. [Google Scholar] [CrossRef]
- Lam, K.H.S.; Lai, W.W.; Ngai, H.Y.; Wu, W.K.R.; Wu, Y.T. Commentary: Ultrasound-Guided Triamcinolone Acetonide Hydrodissection for Carpal Tunnel Syndrome: A Randomized Controlled Trial. Front. Med. 2021, 8, 833862. [Google Scholar] [CrossRef]
- Lam, K.H.S.; Lai, W.W.; Ngai, H.Y.; Wu, W.K.R.; Wu, Y.T. Comment on the safety of the ultrasound-guided hydrodissection technique for carpal tunnel syndrome. J. Ultrasound 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Ho, T.Y.; Chou, Y.C.; Ke, M.J.; Li, T.Y.; Tsai, C.K.; Chen, L.C. Six-month Efficacy of Perineural Dextrose for Carpal Tunnel Syndrome: A Prospective, Randomized, Double-Blind, Controlled Trial. Mayo Clin. Proc. 2017, 92, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.H.; Reeves, K.D.; Cheng, A.L. Transition from deep regional blocks toward deep nerve hydrodissection in the upper body and torso: Method description and results from a retrospective chart review of the analgesic effect of 5% dextrose water as the primary hydrodissection injectate to enhance safety. BioMed Res. Int. 2017, 2017, 7920438. [Google Scholar] [PubMed]
- Wu, Y.T.; Ke, M.J.; Ho, T.Y.; Li, T.Y.; Shen, Y.P.; Chen, L.C. Randomized double-blinded clinical trial of 5% dextrose versus triamcinolone injection for carpal tunnel syndrome patients. Ann. Neurol. 2018, 84, 601–610. [Google Scholar] [CrossRef]
- Lin, M.T.; Liao, C.L.; Hsiao, M.Y.; Hsueh, H.W.; Chao, C.C.; Wu, C.H. Volume Matters in Ultrasound-Guided Perineural Dextrose Injection for Carpal Tunnel Syndrome: A Randomized, Double-Blinded, Three-Arm Trial. Front. Pharmacol. 2020, 11, 625830. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Liu, I.C.; Syu, W.T.; Kuo, P.L.; Wu, C.H. Effect of Perineural Injection with Different Dextrose Volumes on Median Nerve Size, Elasticity and Mobility in Hands with Carpal Tunnel Syndrome. Diagnostics 2021, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Chen, S.R.; Shen, Y.P.; Chang, C.Y.; Su, Y.C.; Chen, L.C.; Wu, Y.T. Long-term outcome after perineural injection with 5% dextrose for carpal tunnel syndrome: A retrospective follow-up study. Rheumatology 2021, 60, 881–887. [Google Scholar] [CrossRef]
- Chen, L.C.; Ho, T.Y.; Shen, Y.P.; Su, Y.C.; Li, T.Y.; Tsai, C.K.; Wu, Y.T. Perineural Dextrose and Corticosteroid Injections for Ulnar Neuropathy at the Elbow: A Randomized Double-blind Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1296–1303. [Google Scholar] [CrossRef]
- Kothari, M.J.J.U.; Waltham, M.A. Disponível em: Carpal Tunnel Syndrome: Treatment and Prognosis. 2019. Available online: https://www.uptodate.com/contents/carpal-tunnel-syndrome-treatment--and-prognosis (accessed on 29 May 2022).
- Amato, A.A.; Barohn, R.J. Peripheral Neuropathy. In Harrison’s Principles of Internal Medicine, 20e; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Wu, Y.T.; Wu, C.H.; Lin, J.A.; Su, D.C.; Hung, C.Y.; Lam, S.K.H. Efficacy of 5% Dextrose Water Injection for Peripheral Entrapment Neuropathy: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 12358. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Kao, C.C.; Yang, M.H.; Fan, G.Y.; Cherng, J.H.; Tsao, C.W.; Wu, S.T.; Cha, T.L.; Meng, E. A Novel Intravesical Dextrose Injection Improves Lower Urinary Tract Symptoms on Interstitial Cystitis/Bladder Pain Syndrome. Front. Pharmacol. 2021, 12, 755615. [Google Scholar] [CrossRef]
- Johnston, E.; Kou, Y.; Junge, J.; Chen, L.; Kochan, A.; Johnston, M.; Rabago, D. Hypertonic Dextrose Stimulates Chondrogenic Cells to Deposit Collagen and Proliferate. Cartilage 2021, 13 (Suppl. S2), 213s–224s. [Google Scholar] [CrossRef]
- Pan, P.J.; Wang, J.C.; Tsai, C.C.; Kuo, H.C. Identification of early response to hypertonic dextrose prolotherapy markers in knee osteoarthritis patients by an inflammation-related cytokine array. J. Chin. Med. Assoc. 2022, 85, 525–531. [Google Scholar] [CrossRef]
- Wu, Y.T.; Chen, S.R.; Li, T.Y.; Ho, T.Y.; Shen, Y.P.; Tsai, C.K.; Chen, L.C. Nerve hydrodissection for carpal tunnel syndrome: A prospective, randomized, double-blind, controlled trial. Muscle Nerve 2019, 59, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Lam, K.H.S.; Wu, Y.T. Dynamic Ultrasound for Carpal Tunnel Syndrome Caused by Squeezed Median Nerve between the Flexor Pollicis Longus and Flexor Digitorum Tendons. Pain Med. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- MacIver, M.B.; Tanelian, D.L. Activation of C fibers by metabolic perturbations associated with tourniquet ischemia. Anesthesiology 1992, 76, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.F.; Mølck, A.M.; Bøgh, I.B.; Lykkesfeldt, J. Effect of insulin-induced hypoglycaemia on the peripheral nervous system: Focus on adaptive mechanisms, pathogenesis and histopathological changes. J. Neuroendocrinol. 2014, 26, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Burdakov, D.; Jensen, L.T.; Alexopoulos, H.; Williams, R.H.; Fearon, I.M.; O’Kelly, I.; Gerasimenko, O.; Fugger, L.; Verkhratsky, A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 2006, 50, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Laiguillon, M.C.; Courties, A.; Houard, X.; Auclair, M.; Sautet, A.; Capeau, J.; Fève, B.; Berenbaum, F.; Sellam, J. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: Toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1513–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiem, K.; Keating, S.T.; Netea, M.G.; Riksen, N.P.; Tack, C.J.; van Diepen, J.; Stienstra, R. Hyperglycemic Memory of Innate Immune Cells Promotes In Vitro Proinflammatory Responses of Human Monocytes and Murine Macrophages. J. Immunol. 2021, 206, 807–813. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, H.; Wei, T.; Wang, D.; Xia, H.; Zhao, L.; Ji, J.; Gao, H. High Glucose-Induced PC12 Cell Death by Increasing Glutamate Production and Decreasing Methyl Group Metabolism. BioMed Res. Int. 2016, 2016, 4125731. [Google Scholar] [CrossRef] [Green Version]
- Najafi, R.; Sharifi, A.M.; Hosseini, A. Protective effects of alpha lipoic acid on high glucose-induced neurotoxicity in PC12 cells. Metab. Brain Dis. 2015, 30, 731–738. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Liu, S.Y.; Sun, Q.Y.; Ren, J.; Liu, H.X.; Li, H. Proanthocyanidin B2 attenuates high-glucose-induced neurotoxicity of dorsal root ganglion neurons through the PI3K/Akt signaling pathway. Neural Regen. Res. 2018, 13, 1628–1636. [Google Scholar]
- Cho, S.J.; Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Fernando, P.; Zhen, A.X.; Hyun, Y.J.; Ahn, M.J.; Kang, H.K.; Hyun, J.W. 7,8-Dihydroxyflavone Protects High Glucose-Damaged Neuronal Cells against Oxidative Stress. Biomol. Ther. 2019, 27, 85–91. [Google Scholar] [CrossRef]
- Cao, M.; Jiang, J.; Du, Y.; Yan, P. Mitochondria-targeted antioxidant attenuates high glucose-induced P38 MAPK pathway activation in human neuroblastoma cells. Mol. Med. Rep. 2012, 5, 929–934. [Google Scholar] [CrossRef]
- Wohnsland, S.; Bürgers, H.F.; Kuschinsky, W.; Maurer, M.H. Neurons and neuronal stem cells survive in glucose-free lactate and in high glucose cell culture medium during normoxia and anoxia. Neurochem. Res. 2010, 35, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Y.; Fan, J.; Yu, L.H.; Ma, B.; Cheng, L.M. The Up-regulation of TNF-α Maintains Trigeminal Neuralgia by Modulating MAPKs Phosphorylation and BKCa Channels in Trigeminal Nucleus Caudalis. Front. Cell. Neurosci. 2021, 15, 764141. [Google Scholar] [CrossRef]
- Peng, Y.; Chu, S.; Yang, Y.; Zhang, Z.; Pang, Z.; Chen, N. Neuroinflammatory In Vitro Cell Culture Models and the Potential Applications for Neurological Disorders. Front. Pharmacol. 2021, 12, 671734. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.M.; Fang, G.X.; Xie, X.M.; Liu, L.H.; Yan, J.; Liu, D.B.; Yu, H.Y. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1524–1536. [Google Scholar]
- Wang, S.; Xia, B.; Qiao, Z.; Duan, L.; Wang, G.; Meng, W.; Liu, Z.; Wang, Y.; Zhang, M. Tetramethylpyrazine attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells through regulating apoptosis, autophagy and oxidative damage. Drug Des. Dev. Ther. 2019, 13, 1187–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Huang, H.; Li, R.; Bi, W.; Feng, L.; E, L.; Hu, M.; Wen, W. Mitophagy protects SH-SY5Y neuroblastoma cells against the TNFα-induced inflammatory injury: Involvement of microRNA-145 and Bnip3. Biomed. Pharmacother. 2019, 109, 957–968. [Google Scholar] [CrossRef]
- Carling, D. The AMP-activated protein kinase cascade—A unifying system for energy control. Trends Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef]
- Wang, S.; Dai, Y. Roles of AMPK and Its Downstream Signals in Pain Regulation. Life 2021, 11, 836. [Google Scholar] [CrossRef]
- Rabago, D.; Patterson, J.J.; Mundt, M.; Kijowski, R.; Grettie, J.; Segal, N.A.; Zgierska, A. Dextrose prolotherapy for knee osteoarthritis: A randomized controlled trial. Ann. Fam. Med. 2013, 11, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, H.; Kyriazis, M.; Reeves, K.D.; Lyftogt, J.; Rabago, D. Topical Mannitol Reduces Capsaicin-Induced Pain: Results of a Pilot-Level, Double-Blind, Randomized Controlled Trial. PM&R 2015, 7, 1111–1117. [Google Scholar]
- Zamami, Y.; Takatori, S.; Yamawaki, K.; Miyashita, S.; Mio, M.; Kitamura, Y.; Kawasaki, H. Acute hyperglycemia and hyperinsulinemia enhance adrenergic vasoconstriction and decrease calcitonin gene-related peptide-containing nerve-mediated vasodilation in pithed rats. Hypertens. Res. 2008, 31, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Wang, L.; Han, J.; Song, J.; Yao, L.; Shao, L.; Sun, Z.; Zheng, L. Decreased expression of transient receptor potential vanilloid 1 impaires the postischemic recovery of diabetic mouse hearts. Circ. J. 2009, 73, 1127–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, M.; Gosu, V.; Basith, S.; Hong, S.; Choi, S. Polymodal Transient Receptor Potential Vanilloid Type 1 Nocisensor: Structure, Modulators, and Therapeutic Applications. Adv. Protein Chem. Struct. Biol. 2016, 104, 81–125. [Google Scholar]
- Cámara-Lemarroy, C.R.; Guzmán-de la Garza, F.J.; Fernández-Garza, N.E. Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. Neuroimmunomodulation 2010, 17, 314–324. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Chen, Q.; M, A.E.; Pal, A.; Eguchi, S.; Wu, L.; Ma, D. The role of microglia in the pathobiology of neuropathic pain development: What do we know? Br. J. Anaesth. 2017, 118, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Fusco, M.; Skaper, S.D.; Coaccioli, S.; Varrassi, G.; Paladini, A. Degenerative Joint Diseases and Neuroinflammation. Pain Pract. 2017, 17, 522–532. [Google Scholar] [CrossRef]
- Wang, H.; Ahrens, C.; Rief, W.; Gantz, S.; Schiltenwolf, M.; Richter, W. Influence of depression symptoms on serum tumor necrosis factor-alpha of patients with chronic low back pain. Arthritis Res. Ther. 2010, 12, R186. [Google Scholar] [CrossRef] [Green Version]
- Park, H.H.; Lo, Y.C.; Lin, S.C.; Wang, L.; Yang, J.K.; Wu, H. The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu. Rev. Immunol. 2007, 25, 561–586. [Google Scholar] [CrossRef] [Green Version]
- Pregi, N.; Wenker, S.; Vittori, D.; Leirós, C.P.; Nesse, A. TNF-alpha-induced apoptosis is prevented by erythropoietin treatment on SH-SY5Y cells. Exp. Cell Res. 2009, 315, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Park, J.G.; Yuk, Y.; Rhim, H.; Yi, S.Y.; Yoo, Y.S. Role of p38 MAPK in the regulation of apoptosis signaling induced by TNF-alpha in differentiated PC12 cells. J. Biochem. Mol. Biol. 2002, 35, 267–272. [Google Scholar] [PubMed] [Green Version]
- Prajapati, P.; Sripada, L.; Singh, K.; Bhatelia, K.; Singh, R.; Singh, R. TNF-alpha regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim. Biophys. Acta 2015, 1852, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.J.; Zhang, J.G.; Wu, L. Plumbagin inhibits neuronal apoptosis, intimal hyperplasia and also suppresses TNF-alpha/NF-kappaB pathway induced inflammation and matrix metalloproteinase-2/9 expression in rat cerebral ischemia. Saudi J. Biol. Sci. 2018, 25, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Rajchgot, T.; Thomas, S.C.; Wang, J.C.; Ahmadi, M.; Balood, M.; Crosson, T.; Dias, J.P.; Couture, R.; Claing, A. Neurons and Microglia; A Sickly-Sweet Duo in Diabetic Pain Neuropathy. Front. Neurosci. 2019, 13, 25. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.; Wang, X.; Liu, L.; Huang, D.; Zhang, R.; Guo, L.; Wang, Z.; Li, X.; Liu, G.; et al. High levels of acetoacetate and glucose increase expression of cytokines in bovine hepatocytes, through activation of the NF-kappaB signalling pathway. J. Dairy Res. 2016, 83, 51–57. [Google Scholar] [CrossRef]
- Nagai, K.; Fukushima, T.; Oike, H.; Kobori, M. High glucose increases the expression of proinflammatory cytokines and secretion of TNFalpha and beta-hexosaminidase in human mast cells. Eur. J. Pharmacol. 2012, 687, 39–45. [Google Scholar] [CrossRef]
- Shanmugam, N.; Reddy, M.A.; Guha, M.; Natarajan, R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 2003, 52, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Grosick, R.; Alvarado-Vazquez, P.A.; Messersmith, A.R.; Romero-Sandoval, E.A. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J. Pain Res. 2018, 11, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, Y.; Herrera, M.T.; Soldevila, G.; Garcia-Garcia, L.; Fabian, G.; Perez-Armendariz, E.M.; Bobadilla, K.; Guzman-Beltran, S.; Sada, E. High glucose concentrations induce TNF-alpha production through the down-regulation of CD33 in primary human monocytes. BMC Immunol. 2012, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Park, K.M.; Bowers, W.J. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell. Signal. 2010, 22, 977–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fregnan, F.; Muratori, L.; Simões, A.R.; Giacobini-Robecchi, M.G.; Raimondo, S. Role of inflammatory cytokines in peripheral nerve injury. Neural Regen. Res. 2012, 7, 2259–2266. [Google Scholar] [PubMed]
- Gumy, L.F.; Bampton, E.T.; Tolkovsky, A.M. Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol. Cell. Neurosci. 2008, 37, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Tumor necrosis factor alpha-induced inflammation is increased but apoptosis is inhibited by common food additive carrageenan. J. Biol. Chem. 2010, 285, 39511–39522. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, S.; Tanabe, K.; Takai, S.; Matsushima-Nishiwaki, R.; Adachi, S.; Iida, H.; Kozawa, O.; Dohi, S. Involvement of Rho-kinase in tumor necrosis factor-alpha-induced interleukin-6 release from C6 glioma cells. Neurochem. Int. 2009, 55, 438–445. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Cavallucci, V.; Middei, S.; Marchetti, C.; Pacioni, S.; Ferri, A.; Diamantini, A.; De Zio, D.; Carrara, P.; Battistini, L.; et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2011, 14, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Micheau, O. Regulation of TNF-Related Apoptosis-Inducing Ligand Signaling by Glycosylation. Int. J. Mol. Sci. 2018, 19, 715. [Google Scholar] [CrossRef] [Green Version]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Li, D.; Fu, J.; Sun, Y.; Li, Y.; Qu, R.; Jin, X.; Li, D. Upregulation of cyclooxygenase-2 is associated with activation of the alternative nuclear factor kappa B signaling pathway in colonic adenocarcinoma. Am. J. Transl. Res. 2015, 7, 1612–1620. [Google Scholar] [PubMed]
- Pamir, N.; McMillen, T.S.; Kaiyala, K.J.; Schwartz, M.W.; LeBoeuf, R.C. Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology 2009, 150, 4124–4134. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.H.; Naruishi, K.; Kajiura, Y.; Nishikawa, Y.; Ikuta, T.; Kido, J.I.; Nagata, T. High Glucose-Mediated Cytokine Regulation in Gingival Fibroblasts and THP-1 Macrophage: A Possible Mechanism of Severe Periodontitis with Diabetes. Cell. Physiol. Biochem. 2018, 50, 973–986. [Google Scholar] [CrossRef]
- Wieman, H.L.; Wofford, J.A.; Rathmell, J.C. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol. Biol. Cell 2007, 18, 1437–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikhman, A.R.; Brinson, D.C.; Valbracht, J.; Lotz, M.K. Cytokine regulation of facilitated glucose transport in human articular chondrocytes. J. Immunol. 2001, 167, 7001–7008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Keller, J.N. Regulation of energy metabolism by inflammation: A feedback response in obesity and calorie restriction. Aging 2010, 2, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Tsui, B.C.; Kropelin, B. The electrophysiological effect of dextrose 5% in water on single-shot peripheral nerve stimulation. Anesth. Analg. 2005, 100, 1837–1839. [Google Scholar] [CrossRef]
- Hashimoto, K.; Sakura, S.; Bollen, A.W.; Ciriales, R.; Drasner, K. Comparative toxicity of glucose and lidocaine administered intrathecally in the rat. Reg. Anesth. Pain Med. 1998, 23, 444–450. [Google Scholar]
- Dufour, E.; Donat, N.; Jaziri, S.; Kurdi, O.; Couturier, C.; Dreyfus, J.F.; Fischler, M. Ultrasound-guided perineural circumferential median nerve block with and without prior dextrose 5% hydrodissection: A prospective randomized double-blinded noninferiority trial. Anesth. Analg. 2012, 115, 728–733. [Google Scholar] [CrossRef]
- Buntragulpoontawee, M.; Chang, K.V.; Vitoonpong, T.; Pornjaksawan, S.; Kitisak, K.; Saokaew, S.; Kanchanasurakit, S. The Effectiveness and Safety of Commonly Used Injectates for Ultrasound-Guided Hydrodissection Treatment of Peripheral Nerve Entrapment Syndromes: A Systematic Review. Front. Pharmacol. 2020, 11, 621150. [Google Scholar] [CrossRef]
- Covey, C.J.; Sineath, M.H., Jr.; Penta, J.F.; Leggit, J.C. Prolotherapy: Can it help your patient? J. Fam. Pract. 2015, 64, 763–768. [Google Scholar] [PubMed]
- Oh, S.; Ettema, A.M.; Zhao, C.; Zobitz, M.E.; Wold, L.E.; An, K.N.; Amadio, P.C. Dextrose-induced subsynovial connective tissue fibrosis in the rabbit carpal tunnel: A potential model to study carpal tunnel syndrome? Hand 2008, 3, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.; Kim, S.S.; Lee, J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yang, W.; Guan, Z.; Yu, W.; Fan, B.; Xu, N.; Liao, D.J. There are only four basic modes of cell death, although there are many ad-hoc variants adapted to different situations. Cell Biosci. 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.E.; Deshmukh, M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat. Cell Biol. 2008, 10, 1477–1483. [Google Scholar] [CrossRef]
- Rea, H.; Kirby, B. A Review of Cutaneous Microdialysis of Inflammatory Dermatoses. Acta Derm. Venereol. 2019, 99, 945–952. [Google Scholar] [CrossRef] [Green Version]
| IL-6 | 5′-TAGCCCTGAGAAAGGAGACATG-3′ | 5′-AGGCAAGTCTCCTCATTGAATC-3′ |
| NF-κB | 5′- CAAGAAGTCCACAAACAC-3′ | 5′- ACCGATATGTCCTCTTTC -3′ |
| COX-2 | 5′-AACATCGTCAATAGCATTC-3′ | 5′-AACATCGTCAATAGCATTC-3′ |
| IL-1β | 5′-AGAAGCTTCCACCAATACTC-3′ | 5′-AGCACCTAGTTGTAAGGAAG-3′ |
| β-actin | 5′-TGACGTGGACATCCGCAAAG-3′ | 5′-CTGGAAGGTGGACAGCGAGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-T.; Chen, Y.-P.; Lam, K.H.S.; Reeves, K.D.; Lin, J.-A.; Kuo, C.-Y. Mechanism of Glucose Water as a Neural Injection: A Perspective on Neuroinflammation. Life 2022, 12, 832. https://doi.org/10.3390/life12060832
Wu Y-T, Chen Y-P, Lam KHS, Reeves KD, Lin J-A, Kuo C-Y. Mechanism of Glucose Water as a Neural Injection: A Perspective on Neuroinflammation. Life. 2022; 12(6):832. https://doi.org/10.3390/life12060832
Chicago/Turabian StyleWu, Yung-Tsan, Yen-Po Chen, King Hei Stanley Lam, Kenneth Dean Reeves, Jui-An Lin, and Cheng-Yi Kuo. 2022. "Mechanism of Glucose Water as a Neural Injection: A Perspective on Neuroinflammation" Life 12, no. 6: 832. https://doi.org/10.3390/life12060832
APA StyleWu, Y.-T., Chen, Y.-P., Lam, K. H. S., Reeves, K. D., Lin, J.-A., & Kuo, C.-Y. (2022). Mechanism of Glucose Water as a Neural Injection: A Perspective on Neuroinflammation. Life, 12(6), 832. https://doi.org/10.3390/life12060832







