Anti-Obesity Effects of Carnosine Supplementation and Exercise in Mice Fed a High-Fat Diet with Concomitant UCP1 Expression in White Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Diet Groups
2.2. Measurement of Diet and Water Intake, and Body Weight
2.3. Sample Collection and Tissue Preparation
2.4. Exercise Loading Protocol
2.5. Morphological Observations in Epididymal Adipose Tissue
2.6. UCP1 Expression in Epididymal Adipose Tissue by Immunostaining
2.7. UCP1 Gene Expression in Epididymal Adipose Tissue by RT-PCR
2.8. Serum Biochemical Marker
2.9. Statistical Analysis
3. Results
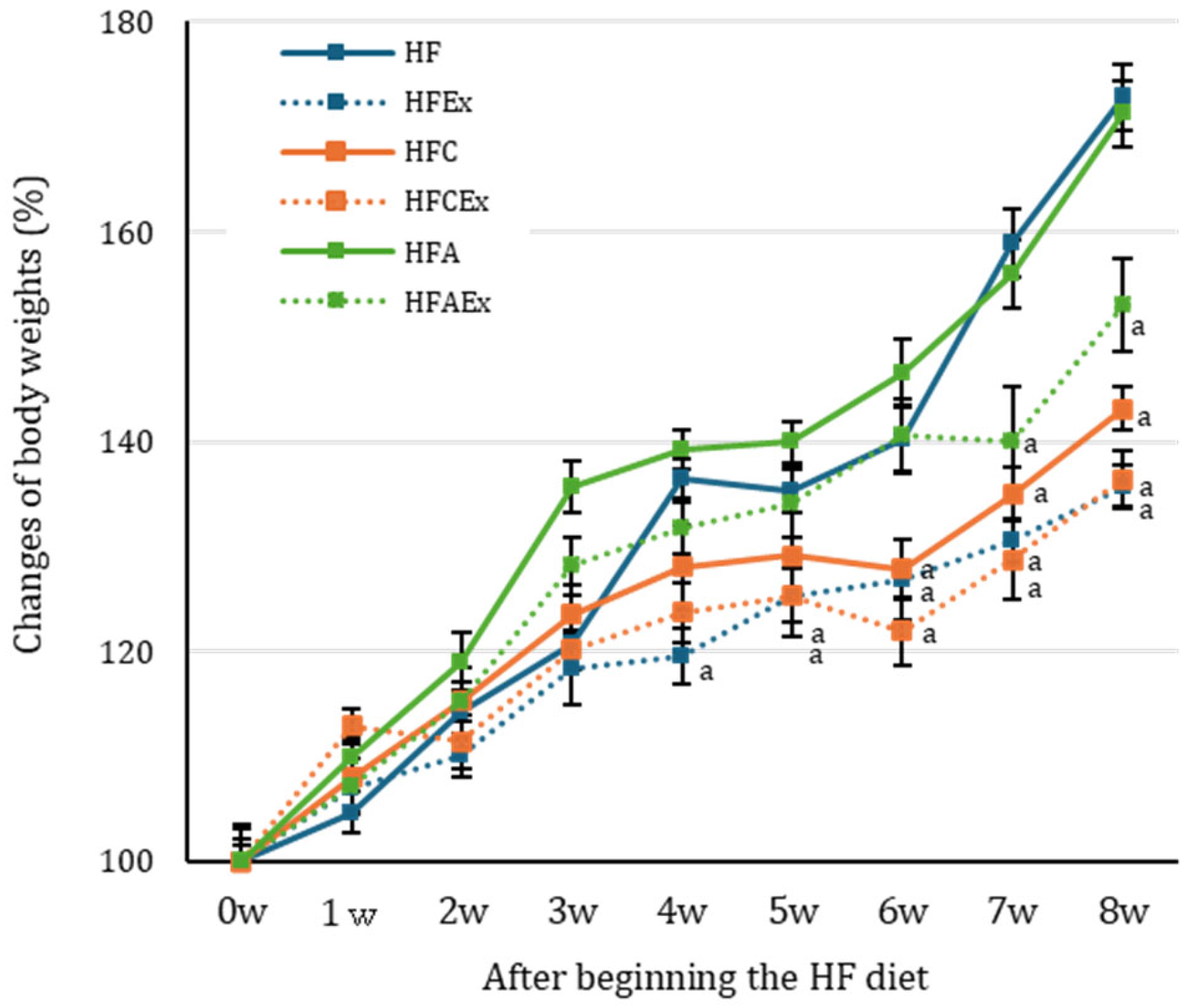
3.1. Body Weight Changes and the HF Diet Intake
3.2. Weight of Visceral Adipose Tissue, Liver and Muscle
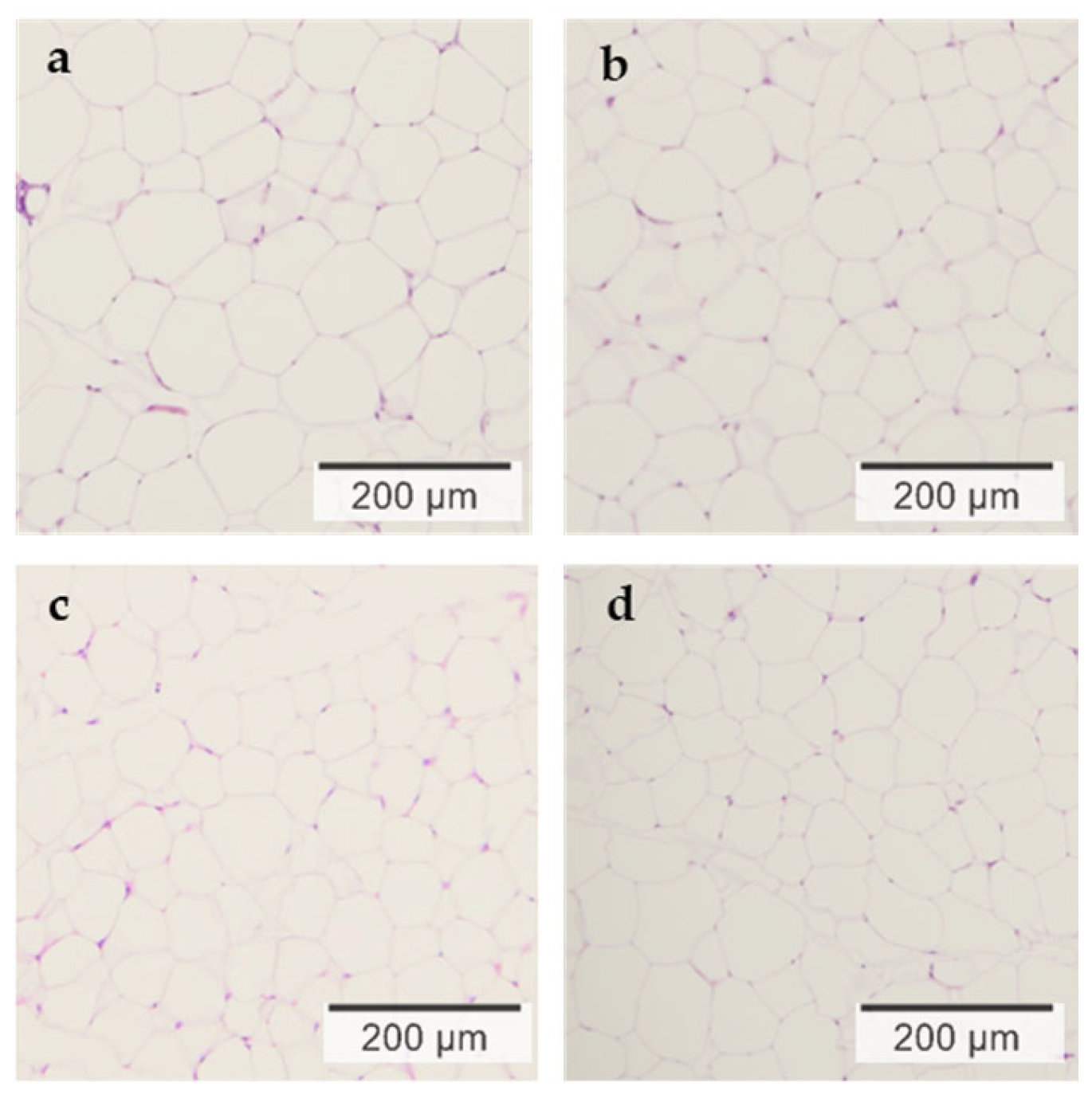
3.3. Morphological Observation of Epididymal Adipose Tissue
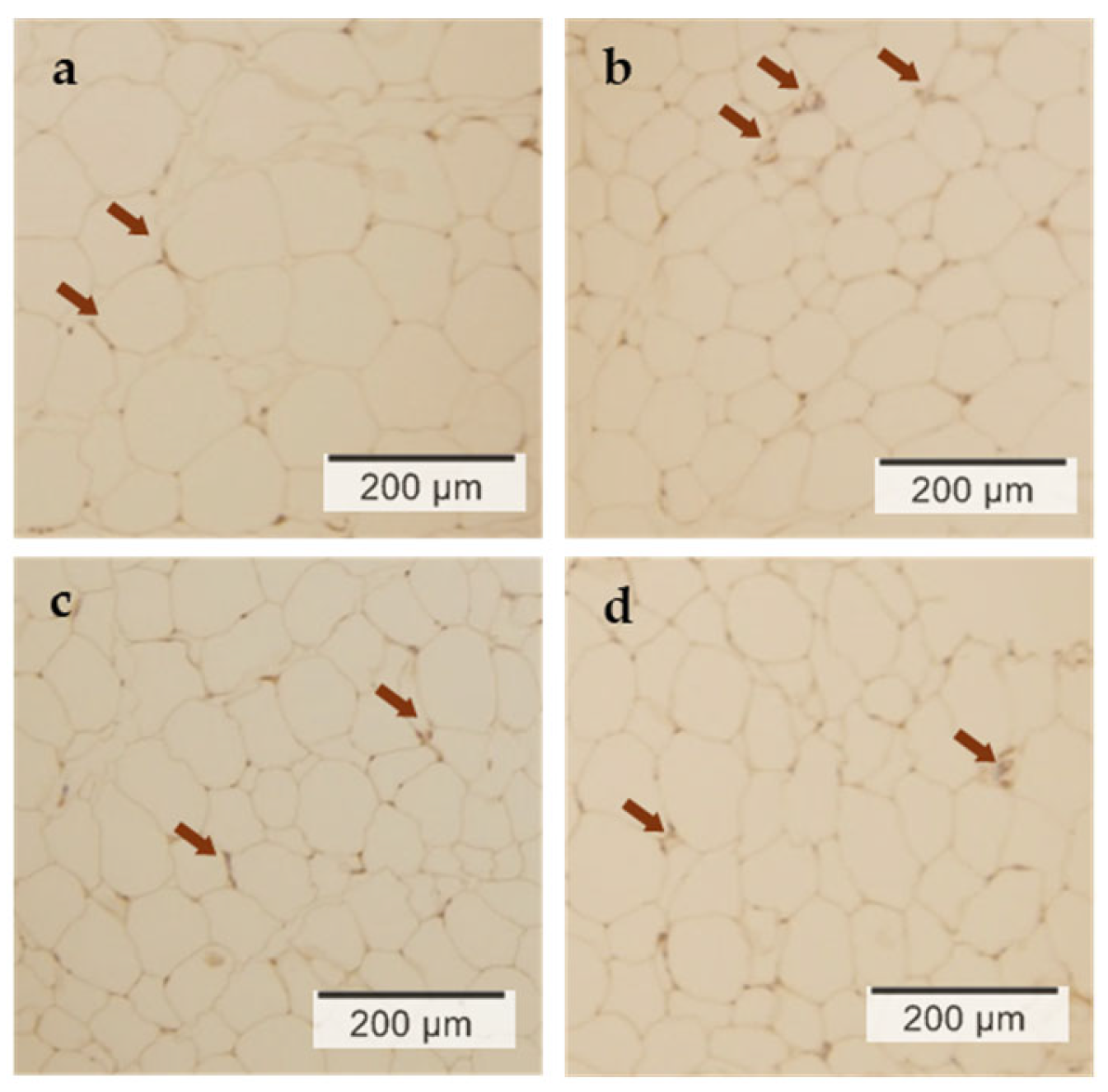
3.4. Histological Expression of UCP1 in Epididymal Adipose Tissue by Immunostaining
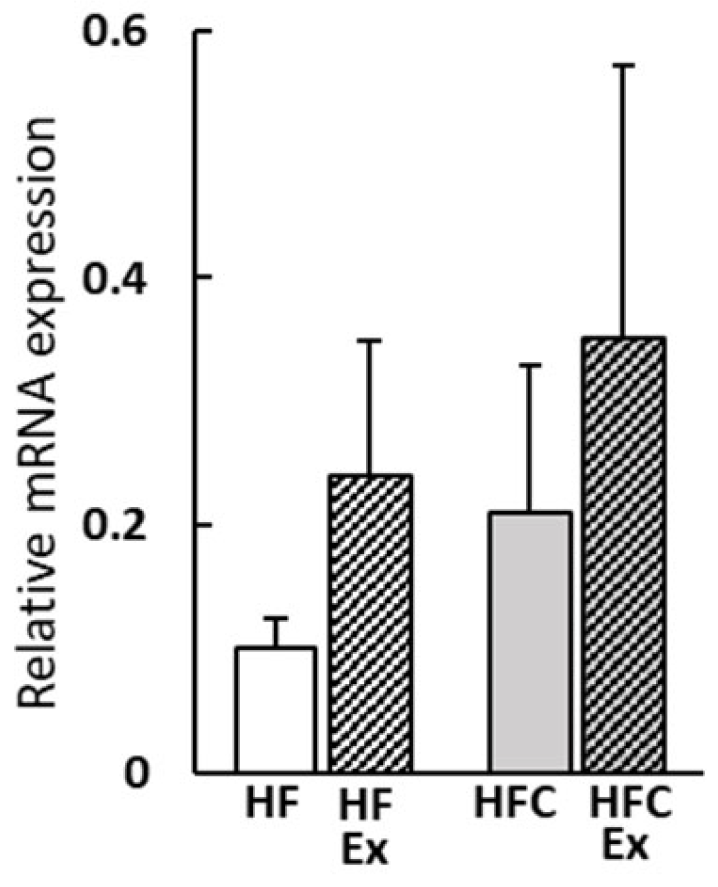
3.5. Gene Expression of UCP1 in Epididymal Adipose Tissue
3.6. Serum Biochemical Parameter
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HF | high fat |
| WAT | white adipose tissue |
| BAT | brown adipose tissue |
| UCP1 | uncoupling protein-1 |
| AIN | American Institute of Nutrition |
| HFEx | HF diet, with exercise |
| HFC | HF diet supplemented carnosine |
| HFA | HF diet supplemented anserine |
| HFCEx | HF diet supplemented carnosine, with exercise |
| HFAEx | HF diet supplemented anserine, with exercise |
| ANOVA | analyses of variance |
| SE | standard errors |
| T-CHO | total cholesterol |
| F-CHO | free cholesterol |
| TG | triglycerides |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| AMPK | AMP-activated protein kinase. |
References
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 31 July 2025).
- Haslam, D.W.; James, W.P.T. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Grundy, S.M. Obesity, Metabolic Syndrome, and Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef]
- Lissner, L.; Heitmann, B.L. Dietary Fat and Obesity: Evidence from Epidemiology. Eur. J. Clin. Nutr. 1995, 49, 79–90. [Google Scholar]
- Cao, H. Adipocytokines in Obesity and Metabolic Disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and Glucose Metabolism in White Adipocytes: Pathways, Dysfunction and Therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef]
- Ballinger, M.A.; Andrews, M.T. Nature’s Fat-Burning Machine: Brown Adipose Tissue in a Hibernating Mammal. J. Exp. Biol. 2018, 221, jeb162586. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected Evidence for Active Brown Adipose Tissue in Adult Humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K.; Yoneshiro, T.; Nio-Kobayashi, J.; Iwanaga, T.; Miyagawa, M.; Kameya, T.; Nakada, K.; et al. High Incidence of Metabolically Active Brown Adipose Tissue in Healthy Adult Humans: Effects of Cold Exposure and Adiposity. Diabetes 2019, 58, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, I.; Vidal-Puig, A. Studying Brown Adipose Tissue in a Human in vitro Context. Front. Endocrinol. 2020, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Prieto, F.J.; Martinez-Tellez, B.; Sanchez-Delgado, G.; Aguilera, C.M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Ruiz, J.R. Activation of Human Brown Adipose Tissue by Capsinoids, Catechins, Ephedrine, and Other Dietary Components: A Systematic Review. Adv. Nutr. 2019, 10, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine Activates Thermogenesis in White and Brown Adipose Tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Jakeman, P.M.; Dunnett, M.; Harris, R.C.; Willan, P.L.T. Carnosine and Anserine Concentrations in the Quadriceps Femoris Muscle of Healthy Humans. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Aldini, G.; de Courten, B.; Regazzoni, L.; Gilardoni, E.; Ferrario, G.; Baron, G.; Altomare, A.; D’amato, A.; Vistoli, G.; Carini, M. Understanding the antioxidant and carbonyl sequestering activity of carnosine: Direct and indirect mechanisms. Free Radic. Res. 2020, 55, 321–330. [Google Scholar] [CrossRef]
- Nagai, K.; Niijima, A.; Yamano, T.; Otani, H.; Okumura, N.; Tsuruoka, N.; Nakai, M.; Kiso, Y. Possible Role of L-Carnosine in the Regulation of Blood Glucose Through Controlling Autonomic Nerves. Exp. Biol. Med. 2003, 228, 1138–1145. [Google Scholar] [CrossRef]
- Caruso, G.; Caraci, F.; Jolivet, R.B. Pivotal Role of Carnosine in the Modulation of Brain Cells Activity: Multimodal Mechanism of Action and Therapeutic Potential in Neurodegenerative Disorders. Prog. Neurobiol. 2019, 175, 35–53. [Google Scholar] [CrossRef]
- Aydın, A.F.; Küçükgergin, C.; Bingül, İ.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M. Effect of Carnosine on Renal Function, Oxidation and Glycation Products in the Kidneys of High-Fat Diet/Streptozotocin-Induced Diabetic Rats. Exp. Clin. Endocrinol. Diabetes 2017, 125, 282–289. [Google Scholar] [CrossRef]
- Albrecht, T.; Schilperoort, M.; Zhang, S.; Braun, J.D.; Qiu, J.; Rodriguez, A.; Pastene, D.O.; Kramer, B.K.; Köppel, H.; Baelde, H.; et al. Carnosine Attenuates the Development of Both Type 2 Diabetes and Diabetic Nephropathy in BTBR ob/ob Mice. Sci. Rep. 2017, 7, 44492. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.; Goodyear, L.J. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes 2015, 64, 3334–3342. [Google Scholar] [CrossRef]
- de Jager, S.; Blancquaert, L.; Van der Stede, T.; Lievens, E.; De Baere, S.; Croubels, S.; Gilardoni, E.; Regazzoni, L.G.; Aldini, G.; Bourgois, J.G.; et al. The ergogenic effect of acute carnosine and anserine supplementation: Dosing, timing, and underlying mechanism. J. Int. Soc. Sports Nutr. 2022, 19, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Maemura, H.; Goto, K.; Yoshioka, T.; Sato, M.; Takahata, Y.; Morimatsu, F.; Takamatsu, K. Effects of Carnosine and Anserine Supplementation on Relatively High Intensity Endurance Performance. Int. J. Sport Health Sci. 2006, 4, 86–94. [Google Scholar] [CrossRef][Green Version]
- Shibuya, T.; Kaburagi, T.; Nagai, R.; Oshiro, S. The Effects of Moderate Exercise on Secretory IgA Production in Mice Depends on Dietary Carbohydrate Intake. J. Clin. Biochem. Nutr. 2015, 57, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-Alanine Supplementation to Improve Exercise Capacity and Performance: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef]
- Matthews, J.J.; Dolan, E.; Swinton, P.A.; Santos, L.; Artioli, G.G.; Turner, M.D.; Elliott-Sale, K.J.; Sale, C. Effect of Carnosine or β-Alanine Supplementation on Markers of Glycemic Control and Insulin Resistance in Humans and Animals: A Systematic Review and Meta-analysis. Adv. Nutr. 2021, 12, 343–362. [Google Scholar] [CrossRef]
- Jin, S.Y.; Lee, J.H.; Kim, H.J.; Park, S.J.; Cho, Y.S. Effects of 4 Weeks of Beta-Alanine Intake on Inflammatory Cytokines after Long-Distance Running Exercise. Exerc. Sci. 2022, 31, 188–196. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; Da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the White Adipose Tissue Regulation: New Insights into Nutritional and Metabolic Relevance in Health and Diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Schirinzi, V.; Poli, C.; Berteotti, C.; Leone, A. Browning of Adipocytes: A Potential Therapeutic Approach. Nutrients 2023, 15, 2229. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosine derivatives: New multifunctional drug-like molecules. Amino Acids 2012, 43, 153–163. [Google Scholar] [CrossRef]
- Peng, W.; Mao, P.; Liu, L.; Chen, K.; Zhong, Y.; Xia, W.; Guo, Q.; Tan, S.C.; Rahmani, J.; Varkaneh, H.K.; et al. Effect of carnosine supplementation on lipid profile, fasting blood glucose, HbA1C and insulin resistance: A systematic review and meta-analysis of long-term randomized controlled trials. Complement. Ther. Med. 2020, 48, 102241. [Google Scholar] [CrossRef]
- Everaert, I.; Mooyaart, A.; Baguet, A.; Zutinic, A.; Baelde, H.; Achten, E.; Taes, Y.; De Heer, E.; Derave, W. Vegetarianism, Female Gender and Increasing Age, but Not CNDP1 Genotype, Are Associated with Reduced Muscle Carnosine Levels in Humans. Amino Acids 2011, 40, 1221–1229. [Google Scholar] [CrossRef]
- Baguet, A.; Everaert, I.; Achten, E.; Thomis, M.; Derave, W. The influence of sex, age and heritability on human skeletal muscle carnosine content. Amino Acids 2011, 43, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Szcześniak, D.; Budzeń, S.; Kopeć, W.; Rymaszewska, J. Anserine and Carnosine Supplementation in the Elderly: Effects on Cognitive Functioning and Physical Capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive Thermogenesis in Adipocytes: Is Beige the New Brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, R.; Lucertini, F.; Guescini, M.; Polidori, E.; Zeppa, S.; Stocchi, V.; Cinti, S. Exercise as a New Physiological Stimulus for Brown Adipose Tissue Activity. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 582–590. [Google Scholar] [CrossRef]
- Terzo, M.; Iantomasi, M.; Tsiani, E. Effects of Resveratrol on Adipocytes: Evidence from In Vitro and In Vivo Studies. Molecules 2024, 29, 5359. [Google Scholar] [CrossRef]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef]
- Everaert, I.; Derave, W. Human Skeletal Muscle Carnosine and Its Function: A Focus on Homeostasis, Muscle Contractility and pH. In Imidazole Dipeptides: Chemistry, Analysis, Function and Effects; Royal Society of Chemistry: London, UK, 2015; pp. 295–312. [Google Scholar]
- Dutka, T.L.; Lamboley, C.R.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. Effects of carnosine on contractile apparatus Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in human skeletal muscle fibers. J. Appl. Physiol. 2012, 112, 728–736. [Google Scholar] [CrossRef]
| Contents | |
|---|---|
| PFC ratios (kcal%) | |
| Protein | 20 |
| Fat | 56 |
| Carbohydrate | 24 |
| Total | 100 |
| kcal/g | 5.1 |
| Ingredients (g) | |
| Casein | 200.0 |
| L-Cystine | 3.0 |
| Corn Starch | - |
| Maltodextrin 10 | 132.0 |
| Sucrose | 100.0 |
| Cellulose, | 50.0 |
| Soybean Oil | 30.0 |
| Lard | 216.7 |
| t-Butylhydroquinone | 0.014 |
| Mineral Mix S10022G 1 | 35.0 |
| Vitamin Mix V10037 1 | 10.0 |
| Choline Bitartrate | 2.5 |
| HF | HFEx | HFC | HFCEx | HFA | HFAEx | |
|---|---|---|---|---|---|---|
| initial body weight (g) | 21.12 ±0.47 | 22.31 ±0.26 | 21.08 ±0.26 | 21.74 ±0.46 | 22.03 ±0.48 | 22.12 ±0.56 |
| final body weight (g) | 37.80 ±1.21 | 29.83 ±0.48 a | 30.14 a ±0.16 | 29.78 a ±0.16 | 37.23 ±1.00 | 34.48 ±0.16 |
| Total intake of diet (kcal) | 577.63 | 543.08 | 581.42 | 557.36 | 582.63 | 561.04 |
| (mg/100 g body weight) | ||||||
| Liver | 2.54 ±0.12 | 2.39 ±0.18 | 2.75 ±0.12 | 2.50 ±0.11 | 2.64 ±0.21 | 2.47 ±0.12 |
| muscles (1) | 0.72 ±0.09 | 0.99 ±0.15 | 0.74 ±0.08 | 0.99 ±0.14 | 0.79 ±0.08 | 088 ±0.09 |
| epidydimal adipose tissue | 5.85 ±0.48 | 3.06 ±0.52 a | 3.66 a ±0.80 | 3.46 a ±0.45 | 5.54 ±0.53 | 4.38 ±0.93 |
| perirenal adipose tissue | 2.20 ±0.23 | 1.31 ±0.26 a | 1.34 a ±0.13 | 1.33 a ±0.14 | 2.11 ±0.17 | 1.91 ±0.43 |
| 2-Way ANOVA (1) | |||||||
|---|---|---|---|---|---|---|---|
| HF | HFEx | HFC | HFCEx | Carnosine | Exercise | Interaction | |
| Area of white adipocytes (μm2) | 8637 ±1050 | 4375 * ±038 | 3859 * ±1185 | 4088 * ±1926 | 0.119 | 0.755 | 0.034 |
| UCP1 expression (counts/ 100 μm2 (1) | 8.1 ±2.4 | 15.7 ±6.2 | 17.9 ±10.9 | 19.5 ±11.0 | 0.039 | 0.398 | 0.058 |
| 2-Way ANOVA (1) | |||||||
|---|---|---|---|---|---|---|---|
| HF | HFEx | HFC | HFCEx | Carnosine | Exercise | Interaction | |
| glucose (mg/dL) | 171.3 ±37.0 | 113.3 * ±6.0 | 127.3 * ±10.4 | 124.7 * ±14.5 | 0.211 | 0.350 | 0.049 |
| T-CHO (mg/dL) | 145.7 ±16.8 | 78.3 ±10.1 | 163.0 ±19.0 | 176.3 ±14.9 | 0.057 | 0.239 | 0.075 |
| F-CHO (mg/dL) | 27.0 ±2.6 | 22.0 ±2.0 | 32.0 ±3.0 | 32.0 ±2.0 | 0.110 | 0.115 | 0.115 |
| TG (mg/dL) | 45.0 ±8.9 | 25.7 ±5.9 * | 30.0 ±2.6 | 32.3 ±9.5 | 0.349 | 0.077 | 0.320 |
| HDL-C (mg/dL) | 37.3 ±13.1 | 69.7 ±9.3 | 81.0 *# ±14.5 | 91.3 *# ±3.8 | 0.005 | 0.227 | 0.035 |
| LDL-C (mg/dL) | 57.0 ±19.0 | 35.7 ±2.0 | 75.7 ±27.8 | 78.7 ±13.0 | 0.028 | 0.399 | 0.290 |
| AST (IU/L) | 137.3 ±19.6 | 156.0 ±70.1 | 149.0 ±54.0 | 110.3 ±10.7 | 0.187 | 0.415 | 0.129 |
| ALT (IU/L) | 28.3 ±7.8 | 40.0 ±29.0 | 33.7 ±12.5 | 24.3 ±1.5 | 0.413 | 0.543 | 0.277 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Haruyama, M.; Murano, S.; Kaburagi, T. Anti-Obesity Effects of Carnosine Supplementation and Exercise in Mice Fed a High-Fat Diet with Concomitant UCP1 Expression in White Adipocytes. Nutraceuticals 2025, 5, 39. https://doi.org/10.3390/nutraceuticals5040039
Shen X, Haruyama M, Murano S, Kaburagi T. Anti-Obesity Effects of Carnosine Supplementation and Exercise in Mice Fed a High-Fat Diet with Concomitant UCP1 Expression in White Adipocytes. Nutraceuticals. 2025; 5(4):39. https://doi.org/10.3390/nutraceuticals5040039
Chicago/Turabian StyleShen, Xun, Moe Haruyama, Shizuka Murano, and Tomoko Kaburagi. 2025. "Anti-Obesity Effects of Carnosine Supplementation and Exercise in Mice Fed a High-Fat Diet with Concomitant UCP1 Expression in White Adipocytes" Nutraceuticals 5, no. 4: 39. https://doi.org/10.3390/nutraceuticals5040039
APA StyleShen, X., Haruyama, M., Murano, S., & Kaburagi, T. (2025). Anti-Obesity Effects of Carnosine Supplementation and Exercise in Mice Fed a High-Fat Diet with Concomitant UCP1 Expression in White Adipocytes. Nutraceuticals, 5(4), 39. https://doi.org/10.3390/nutraceuticals5040039






