Phenolic Compounds and Antioxidant Properties of Puruí (Alibertia edulis, Rubiaceae), an Edible Dark Purple Fruit from the Brazilian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extractions
2.3. Chemical Characterization of A. edulis Extracts
2.4. Antioxidant Capacity of the Fruit Pulp of A. edulis
2.4.1. Determination of Total Phenolics Content (TPC)
2.4.2. Determination of Total Flavonoids Content (TFC)
2.4.3. DPPH Radical Scavenging Assay
2.4.4. ABTS Radical Cation Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Composition of Fruit Pulp Extracts of A. edulis
3.2. Composition of Volatile Concentrate of A. edulis Fruit Pulp
3.3. Antioxidant Capacity of the A. edulis Pulp Fruit
3.3.1. Total Phenolic Content (TPC)
3.3.2. Total Flavonoid Content (TFC)
3.3.3. DPPH and TEAC/ABTS Assays
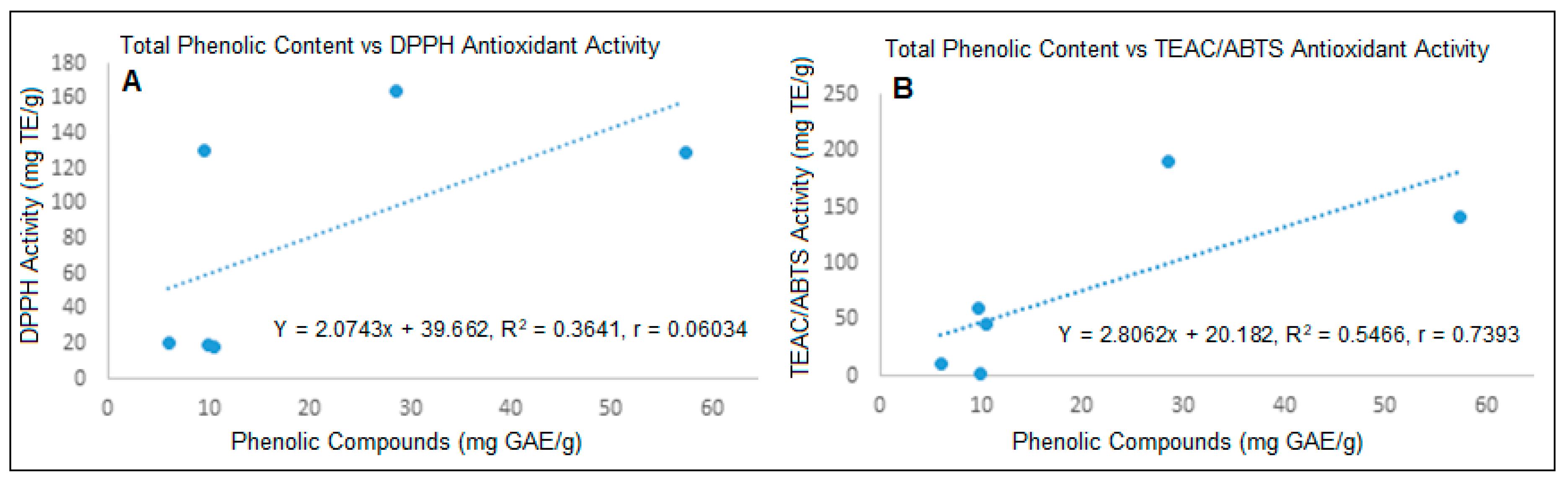
3.3.4. Correlation between Total Phenolic Compounds and Antioxidant Activity of A. edulis Fruit Pulp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bremer, B.; Eriksson, T. Time tree of Rubiaceae: Phylogeny and dating the families, subfmilies, and tribes. Int. J. Plant Sci. 2009, 170, 766–793. [Google Scholar] [CrossRef]
- Delprete, P.G.; Jardim, J.G. Systematics, taxonomy and floristics of Brazilian Rubiaceae: An overview about the current status and future challenges. Rodriguésia 2012, 63, 101–128. [Google Scholar] [CrossRef]
- Persson, C. Phylogeny of the Neotropical Alibertia group (Rubiaceae), with emphasis on the genus of Alibertia, inferred from ITS and 5S ribosomal DNA sequences. Am. J. Bot. 2000, 87, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. 2021. Available online: theplantlist.org/1.1/browse/A/Rubiaceae/Alibertia (accessed on 10 July 2023).
- Cavalcante, P.B. Frutas Comestíveis da Amazônia. Coleção Adolpho Ducke; Museu Paraense Emílio Goeldi: Belém, PA, Brazil, 1996. [Google Scholar]
- Rieder, A. Plants used for diabetes in the transition zone of Platinum and Amazon Hydrographic Basins, Southwest portion of Mato Grosso, Brazil. Planta Medica 2013, 79, PF8. [Google Scholar] [CrossRef]
- Menegati, S.E.L.T.; de Lima, F.F.; Traesel, G.K.; Souza, R.I.C.; dos Santos, A.C.; Aquino, D.F.S.; de Oliveira, V.S.; Vieira, S.C.H.; Cardoso, C.A.L.; Vieira, M.C.; et al. Acute and subacute toxicity of the aqueous extract of Alibertia edulis (Rich.) A. Rich. ex DC. in rats. J. Ethnopharmacol. 2016, 194, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Aquino, D.F.S.; Tirloni, C.A.S.; Menegati, S.E.L.T.; Cardoso, C.A.L.; Vieira, S.C.H.; Vieira, M.C.; Simonet, A.M.; Macías, F.A.; Gasparotto Junior, A. Alibertia edulis (L.C. Rich.) A.C. Rich—A potent diuretic arising from Brazilian indigenous species. J. Ethnopharmacol. 2017, 196, 193–200. [Google Scholar] [CrossRef]
- Aquino, D.F.S.; Monteiro, T.A.; Cardoso, C.A.L.; Vieira, S.C.H.; Vieira, M.C.; Souza, K.P.; Amaya-Farfan, J.; Carvalho, G.C.B.C.; Moura, C.S.; Morato, P.N. Investigation of the antioxidant and hypoglycemiant properties of Alibertia edulis (L.C. Rich.) A.C. Rich. leaves. J. Ethnopharmacol. 2020, 253, 112648. [Google Scholar] [CrossRef]
- Tolouei, S.E.L.; Traesel, G.K.; de Lima, F.F.; de Araújo, F.H.S.; Lescano, C.H.; Cardoso, C.A.L.; Oesterreich, S.A.; Vieira, M.C. Cytotoxic, genotoxic and mutagenic evaluation of Alibertia edulis (rich.) a. Rich. ex DC: An indigenous species from Brazil. Drug Chem. Toxicol. 2018, 43, 200–207. [Google Scholar] [CrossRef]
- Lescano, C.H.; de Lima, F.F.; Cardoso, C.A.L.; Vieira, S.C.H.; Monica, F.Z.; de Oliveira, I.P. Rutin present in Alibertia edulis extract acts on human platelet aggregation through inhibition of cyclooxygenase/thromboxane. Food Funct. 2021, 12, 802–814. [Google Scholar] [CrossRef]
- Brochini, C.B.; Martins, D.; Roque, N.F.; Bolzani, V.S. An oleanane acid from Alibertia edulis. Phytochemistry 1994, 36, 1293–1295. [Google Scholar] [CrossRef]
- da Silva, V.C.; Giannini, M.J.S.M.; Carbone, V.; Piacente, S.; Pizza, C.; Bolzani, V.S.; Lopes, M.N. New antifungal terpenoid glycosides from Alibertia edulis (Rubiaceae). Helv. Chim. Acta 2008, 91, 1355–1362. [Google Scholar] [CrossRef]
- Bolzani, V.S.; Trevisan, L.M.V.; Young, M.C.M. Caffeic acid esters and triterpenes of Alibertia macrophylla. Phytochemistry 1991, 30, 2089–2091. [Google Scholar] [CrossRef]
- Young, M.C.M.; Braga, M.R.; Dietrich, S.M.C.; Gottlieb, H.E.; Trevisan, L.M.V.; Bolzani, V.S. Fungitoxic non-glycosidic iridoids from Alibertia macrophylla. Phytochemistry 1992, 31, 3433–3435. [Google Scholar] [CrossRef]
- da Silva, V.C.; Faria, A.O.; Bolzani, V.S.; Lopes, M.N. A new ent-kaurane diterpene from stems of Alibertia macrophylla. Helv. Chim. Acta 2007, 90, 1781–1785. Available online: http://hdl.handle.net/11449/69936 (accessed on 11 January 2022). [CrossRef]
- Militão, G.C.G.; Pessoa, C.O.; Costa-Lotufo, L.V.; de Moraes, M.E.A.; de Moraes, M.O.; Luciano, J.H.S.; Lima, M.A.S.; Silveira, E.R. Cytotoxicity of flavonoids isolated from Alibertia myrciifolia. Pharm. Biol. 2005, 43, 480–484. [Google Scholar] [CrossRef]
- Luciano, J.H.S.; Lima, M.A.S.; Silveira, E.R.; Vasconcelos, I.M.; Fernandes, G.S.; de Souza, E.B. Antifungal iridoids, triterpenes and phenol compounds from Alibertia myrciifolia Sprunge ex. Schum. Quim. Nova 2010, 33, 292–294. [Google Scholar] [CrossRef]
- Olea, R.S.G.; Roque, N.F.; Bolzani, V.S. Acylated flavonol glycosides and terpenoids from the leaves of Alibertia sessilis. J. Braz. Chem. Soc. 1997, 8, 257–259. [Google Scholar] [CrossRef]
- da Silva, V.C.; Silva, G.H.; Bolzani, V.S.; Lopes, M.N. Isolation of lignans glycosides from Alibertia sessilis (Vell.) K. Schum. (Rubiaceae) by preparative high-performance liquid chromatography. Eclet. Quim. 2006, 31, 55–58. [Google Scholar] [CrossRef]
- Adams, P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Van den Dool, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- da Silva, J.K.R.; Andrade, E.H.A.; Kato, M.J.; Carreira, L.M.M.; Guimarães, E.F.; Maia, J.G.S. Antioxidant capacity and larvicidal and antifungal activities of essential oils and extracts from Piper krukoffii. Nat. Prod. Commun. 2011, 6, 1361–1366. [Google Scholar] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; de Morais, S.M.; Sampaio, C.G.; Pérez-Giménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre ABTS; Comunicado Técnico 128; Embrapa Agroindustrial Tropical: Fortaleza, Brazil, 2007. [Google Scholar]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella Cambess. using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Masike, K.; Mhlong, M.I.; Mudau, S.P.; Nobela, O.; Ncube, E.N.; Tugizimana, S.; George, M.J.; Madala, N.E. Highlighting mass spectrometric fragmentation differences and similarities between hydroxycinnamoyl-quinic acids and hydroxycinnamoyl-isocitric acids. Chem. Cent. J. 2017, 11, 29. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, J.-W.; Zhang, Y.; Zhang, L.-Y.; Zhang, T.; Chen, Y.; Cai, Z.-Z.; Yang, L. Metabolism of genipin in rat and identification of metabolites by using ultraperformance liquid chromatography/quadrupole time-of-flight tandem mass spectrometry. J. Evid. Based Complement. Alternat. Med. 2013, 2013, 957030. [Google Scholar] [CrossRef]
- da Silva, F.M.A.; Koolen, H.H.F.; de Almeida, R.A.; de Souza, A.D.L.; Pinheiro, M.L.B. Desreplicação de alcalóides aporfínicos e oxoaporfínicos de Unonopsis guatteriodes por ESI-IT-MS. Quim. Nova 2012, 35, 944–947. [Google Scholar] [CrossRef]
- Troalen, L.G.; Phillips, A.S.; Peggie, D.A.; Barran, P.E.; Hulme, A.N. Historical textile dyeing with Genista tinctoria L.: A comprehensive study by UPLC-MS/MS analysis. Anal. Meth. 2014, 6, 8915–8923. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Nogueira, G.C.; Vardanega, R.; Meireles, M.A.A. Identification and quantification of genipin and geniposide from Genipa americana L. by HPLC-DAD using a fused-core column. Food Sci. Technol. 2018, 38 (Suppl. 1), 116–122. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.-H.; Smillie, T.J.; Khan, I.A. Identification of phenolic compounds from Scutellaria lateriflora by liquid chromatography with ultraviolet photodiode array and electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 63, 120–127. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zeng, X.; Huang, S.; Hou, S.; Lai, X. Characterization of phenolic constituents in Lithocarpus polystachyus. Anal. Meth. 2014, 6, 1359–1363. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Guimarães, A.C.G.; de Souza Gomes, M.; Zacaroni Lima, L.; Sales, P.F.; da Cunha, M.C.; Rodrigues, L.J.; de Barros, H.E.A.; Pires, C.R.F.; dos Santos, V.F.; Natarelli, C.V.L.; et al. Application of chemometric techniques in the evaluation of bioactive compounds and antioxidant activity of fruit from Brazilian Cerrado. J. Food Meas. Charact. 2023, 17, 2095–2106. [Google Scholar] [CrossRef]
- Becker, F.S. Desenvolvimento, Caracterização e Atividade Antioxidante de Marmelada-de-Cachorro (Alibertia sessilis Schum.). Ph.D. Thesis, Universidade Federal de Lavras, Lavras, MG, Brazil, 2015. [Google Scholar]
- Cani, P.D.; Everard, A. Talking microbes: When gut bacteria interact with diet and host organs. Mol. Nutr. Food Res. 2016, 60, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; Tran, K.; Smith, C.C.; McDonald, M.; Shejwalkar, P.; Hara, K. The Role of the Nrf2/ARE antioxidant system in preventing cardiovascular diseases. Diseases 2016, 4, 34. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 2, e1373208. [Google Scholar] [CrossRef]
- Gregoris, E.; Lima, G.P.P.; Fabris, S.; Bertelle, M.; Sicari, M.; Stevanato, R. Antioxidant properties of Brazilian tropical fruits by correlation between different assays. BioMed Res. Int. 2013, 2013, 132759. [Google Scholar] [CrossRef]

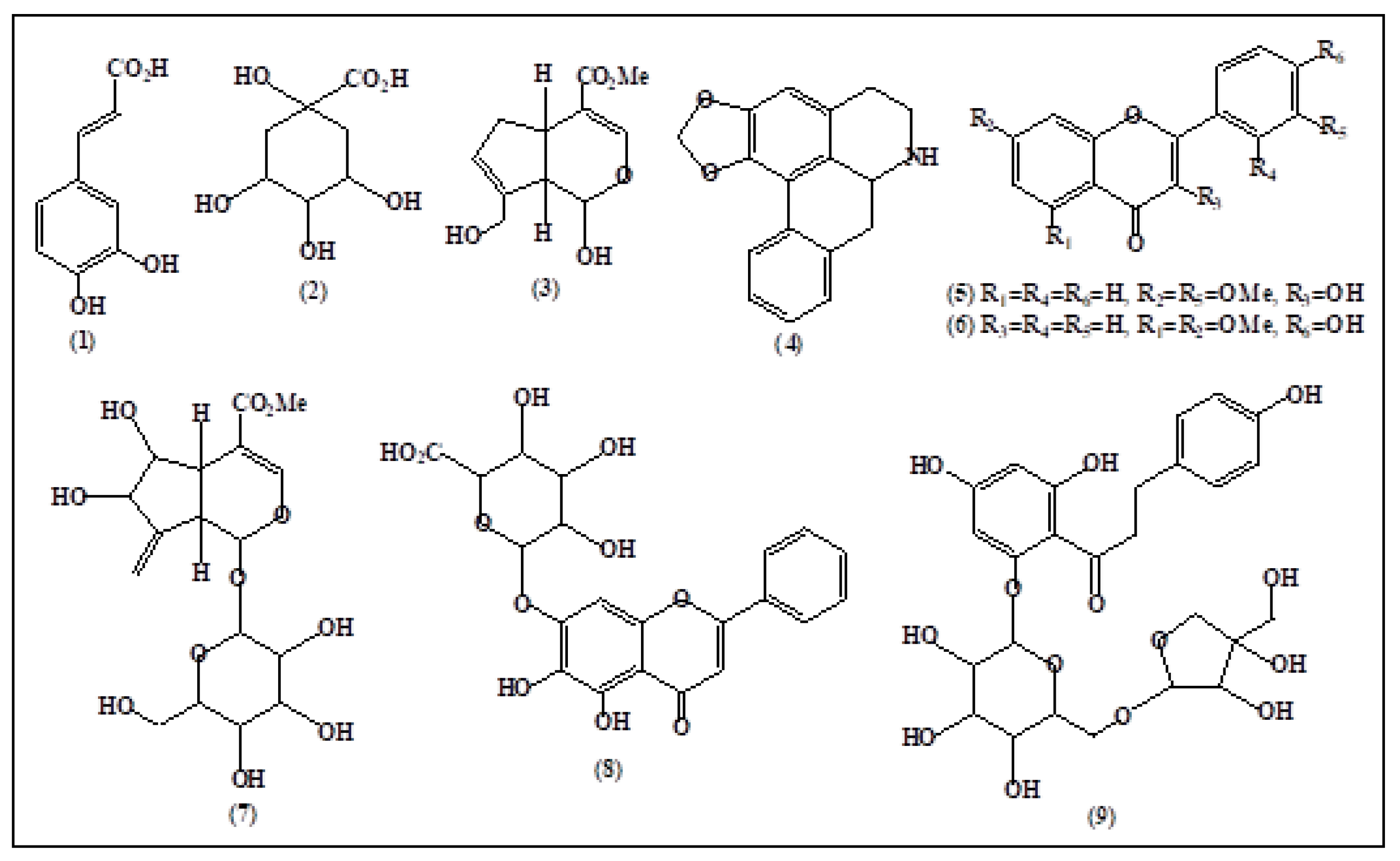
| LPF-E and LPF-A Extracts | Compounds | RT (min) | [M-H]− (m/z) | MSn Fragments (m/z) |
|---|---|---|---|---|
| 1 | Caffeic acid a | 1.7 | 179 | 136 |
| 2 | Quinic acid a | 1.8 | 191 | 173 |
| 3 | Genipin a,b | 10.2 | 225 | 207, 175, 147 |
| 4 | Annonaine a,b | 10.1 | 264 | 249, 219, 191 |
| 5 | 3′,7-Dimethoxy-3-hydroxyflavone a,b | 15.7 | 297 | 265, 149 |
| 6 | 4′-Hydroxy-5,7-dimethoxyflavone a,b | 16.3 | 299 | 281, 255 |
| 7 | 6-Hydroxy-7-epigardoside methyl ester a,b | 6.5 | 403 | 241 |
| 8 | Baicalin a | 18.1 | 445 | 269, 167, 101 |
| 9 | Phloretin-2-O-apiofuranosyl-glucopyranoside a,b | 15.3 | 567 | 419, 273 |
| Constituents | RIC | RIL | LPF-V (%) |
|---|---|---|---|
| Hexanal | 799 | 801 a | 2.1 |
| Furfural | 828 | 828 a | 5.4 |
| (3Z)-Hexenal | 849 | 850 a | 2.2 |
| Heptanal | 901 | 901 a | 0.8 |
| (2E)-Heptenal | 952 | 947 a | 0.4 |
| n-Heptanol | 958 | 959 a | 1.4 |
| Octen-3-ol | 975 | 974 a | 1.9 |
| Benzene acetaldehyde | 1041 | 1036 a | 5.5 |
| γ-Terpinene | 1055 | 1054 a | 0.8 |
| Linalool | 1099 | 1095 a | 2.5 |
| Naphthalene | 1181 | 1178 a | 0.8 |
| Methyl salicylate | 1193 | 1190 a | 0.8 |
| (2E)-Decenal | 1260 | 1260 a | 0.9 |
| Safrole | 1287 | 1285 a | 3.6 |
| Thymol | 1291 | 1289 a | 0.7 |
| Tridecanal | 1511 | 1509 b | 5.4 |
| Palmitic alcohol | 1876 | 1874 a | 5.5 |
| Methyl palmitate | 1925 | 1921 a | 4.8 |
| Palmitic acid | 1962 | 1959 a | 20.4 |
| Ethyl palmitate | 1993 | 1992 b | 0.6 |
| Methyl linoleate | 1386 | 2095 a | 7.1 |
| Methyl linolenate | 1395 | 2098 b | 16.6 |
| Phytol | 1596 | 2106 b | 1.6 |
| Linoleic acid | 1794 | 2132 a | 2.1 |
| Incensole | 2160 | 2158 a | 1.3 |
| Total (%) | 95.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, N.C.C.; Monteiro, O.S.; da Rocha, C.Q.; da Silva, J.K.R.; Maia, J.G.S. Phenolic Compounds and Antioxidant Properties of Puruí (Alibertia edulis, Rubiaceae), an Edible Dark Purple Fruit from the Brazilian Amazon. Nutraceuticals 2023, 3, 529-539. https://doi.org/10.3390/nutraceuticals3040038
Carvalho NCC, Monteiro OS, da Rocha CQ, da Silva JKR, Maia JGS. Phenolic Compounds and Antioxidant Properties of Puruí (Alibertia edulis, Rubiaceae), an Edible Dark Purple Fruit from the Brazilian Amazon. Nutraceuticals. 2023; 3(4):529-539. https://doi.org/10.3390/nutraceuticals3040038
Chicago/Turabian StyleCarvalho, Natale Cristine C., Odair S. Monteiro, Claudia Q. da Rocha, Joyce Kelly R. da Silva, and José Guilherme S. Maia. 2023. "Phenolic Compounds and Antioxidant Properties of Puruí (Alibertia edulis, Rubiaceae), an Edible Dark Purple Fruit from the Brazilian Amazon" Nutraceuticals 3, no. 4: 529-539. https://doi.org/10.3390/nutraceuticals3040038
APA StyleCarvalho, N. C. C., Monteiro, O. S., da Rocha, C. Q., da Silva, J. K. R., & Maia, J. G. S. (2023). Phenolic Compounds and Antioxidant Properties of Puruí (Alibertia edulis, Rubiaceae), an Edible Dark Purple Fruit from the Brazilian Amazon. Nutraceuticals, 3(4), 529-539. https://doi.org/10.3390/nutraceuticals3040038









