Super Fruit Amla (Emblica officinalis, Gaertn) in Diabetes Management and Ensuing Complications: A Concise Review
Abstract
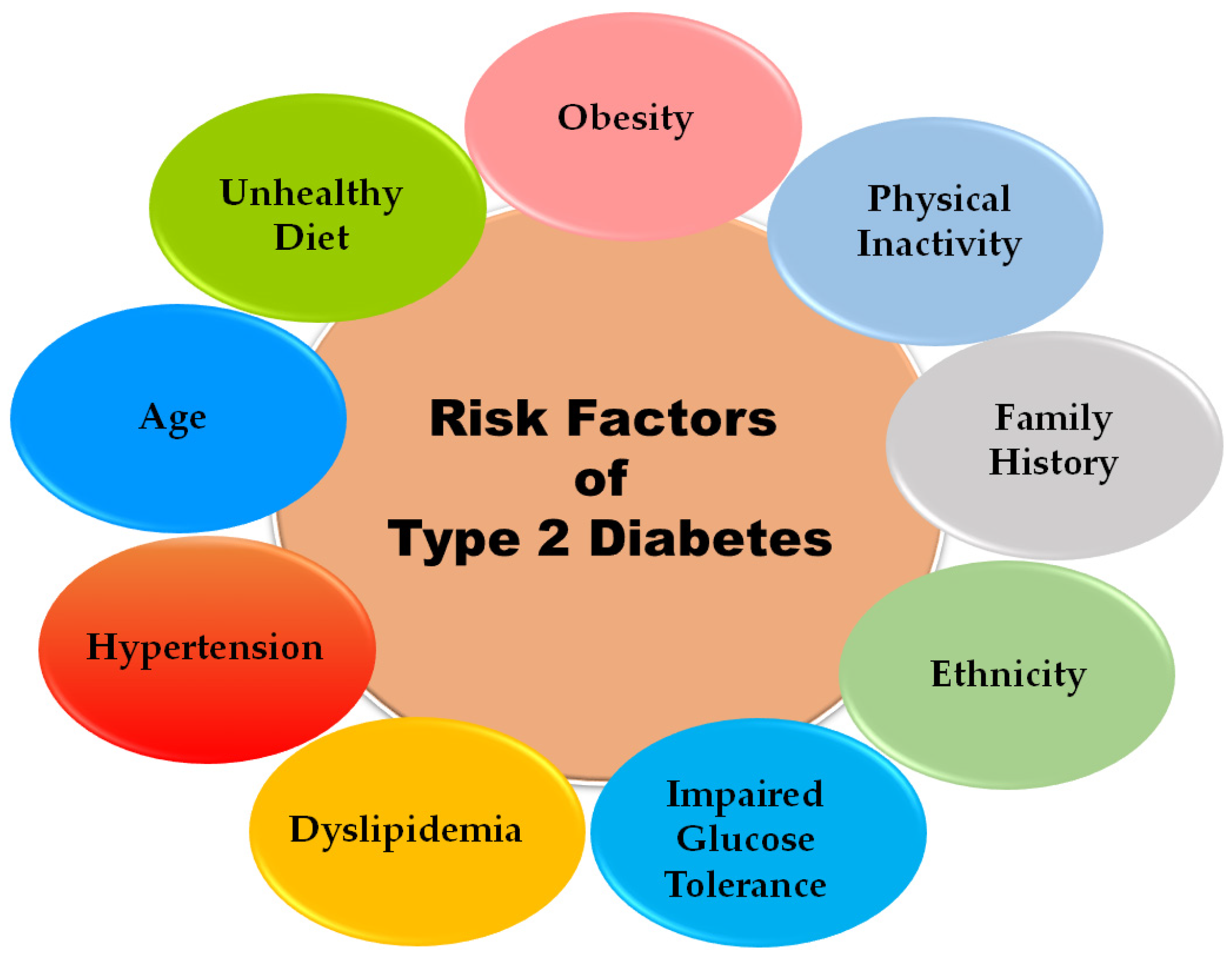
1. Introduction
2. Emblica officinalis (Amla): A Super Fruit for Superior Health
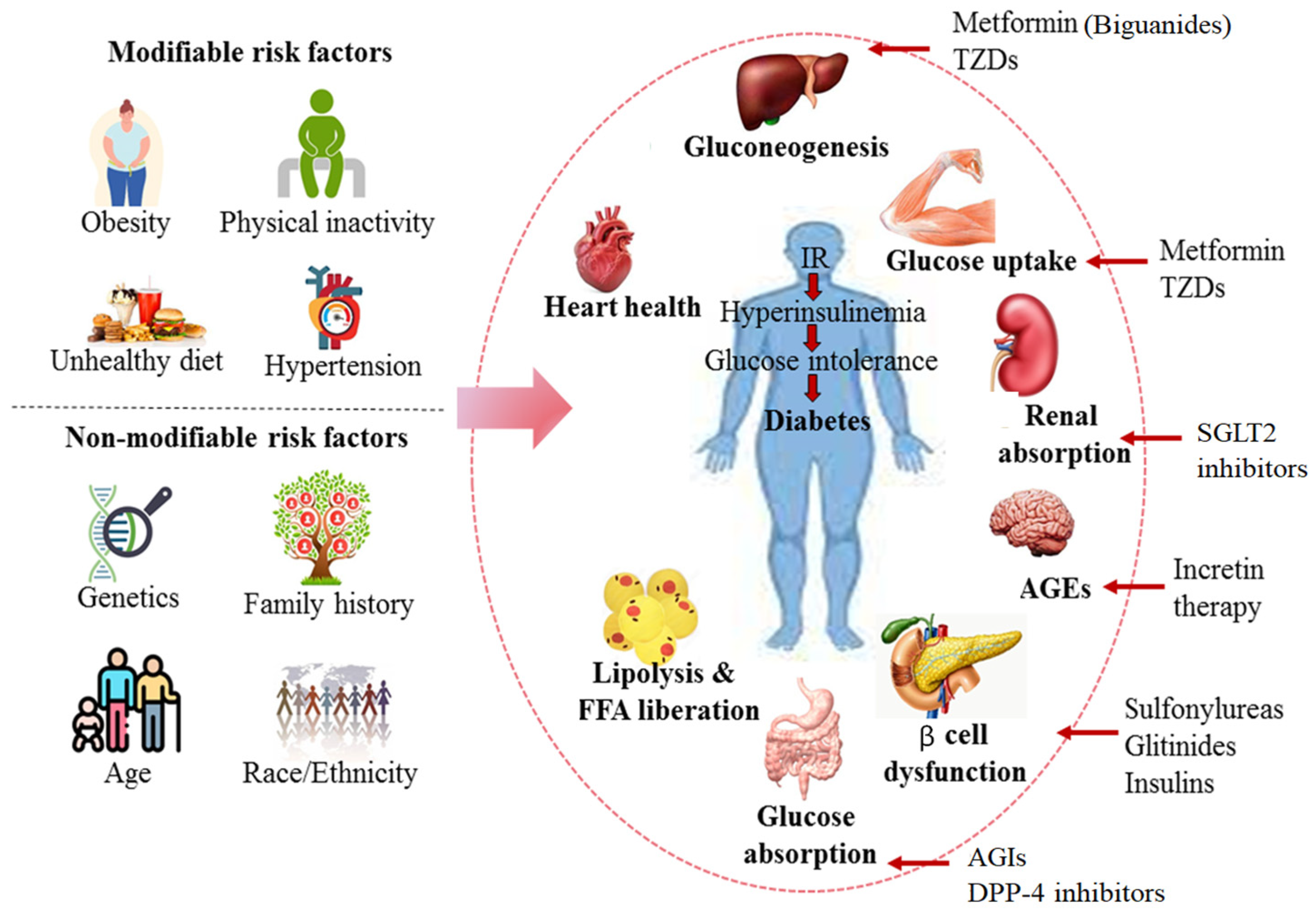
3. Pathophysiology of Diabetes and Drugs Used for Treatment
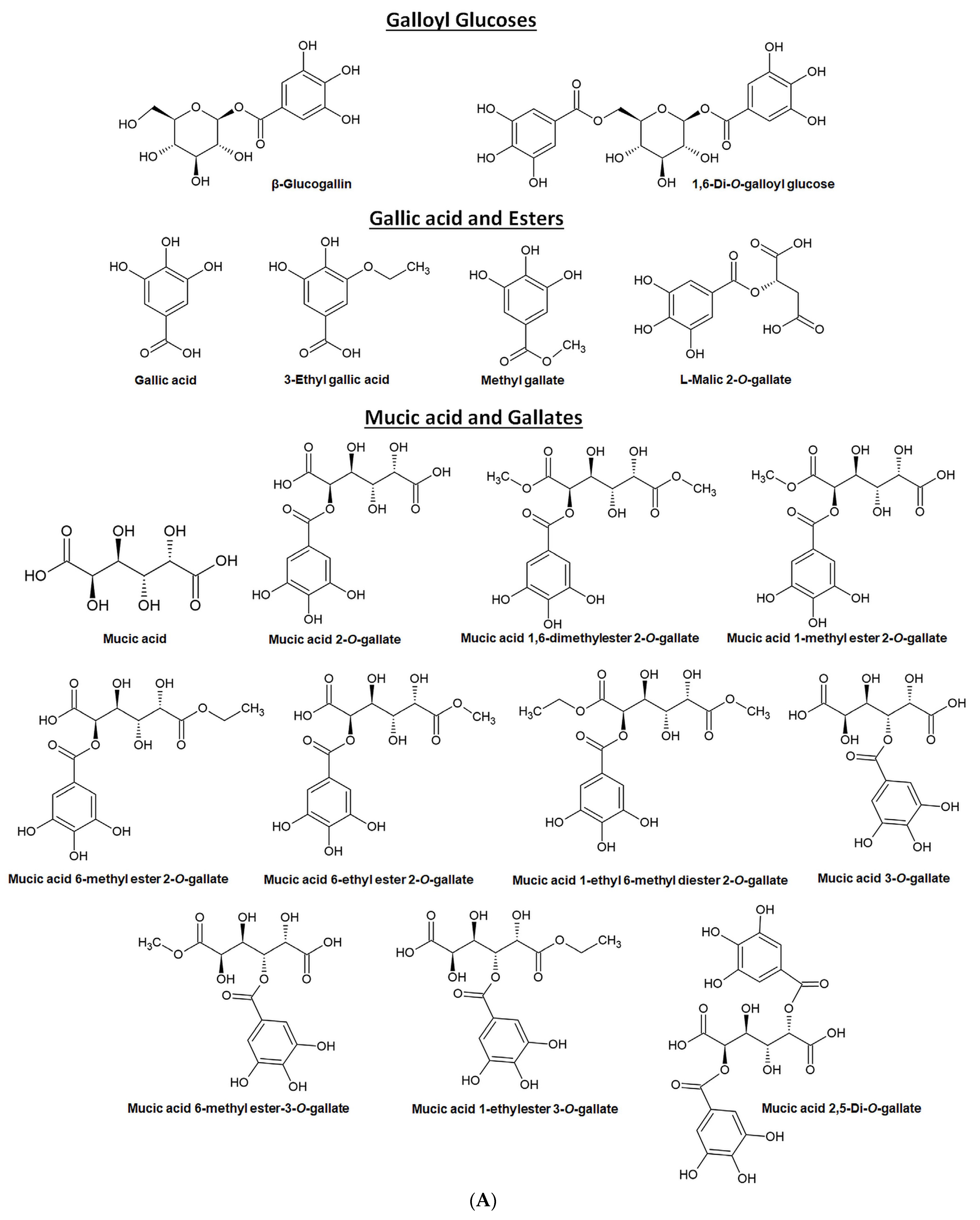
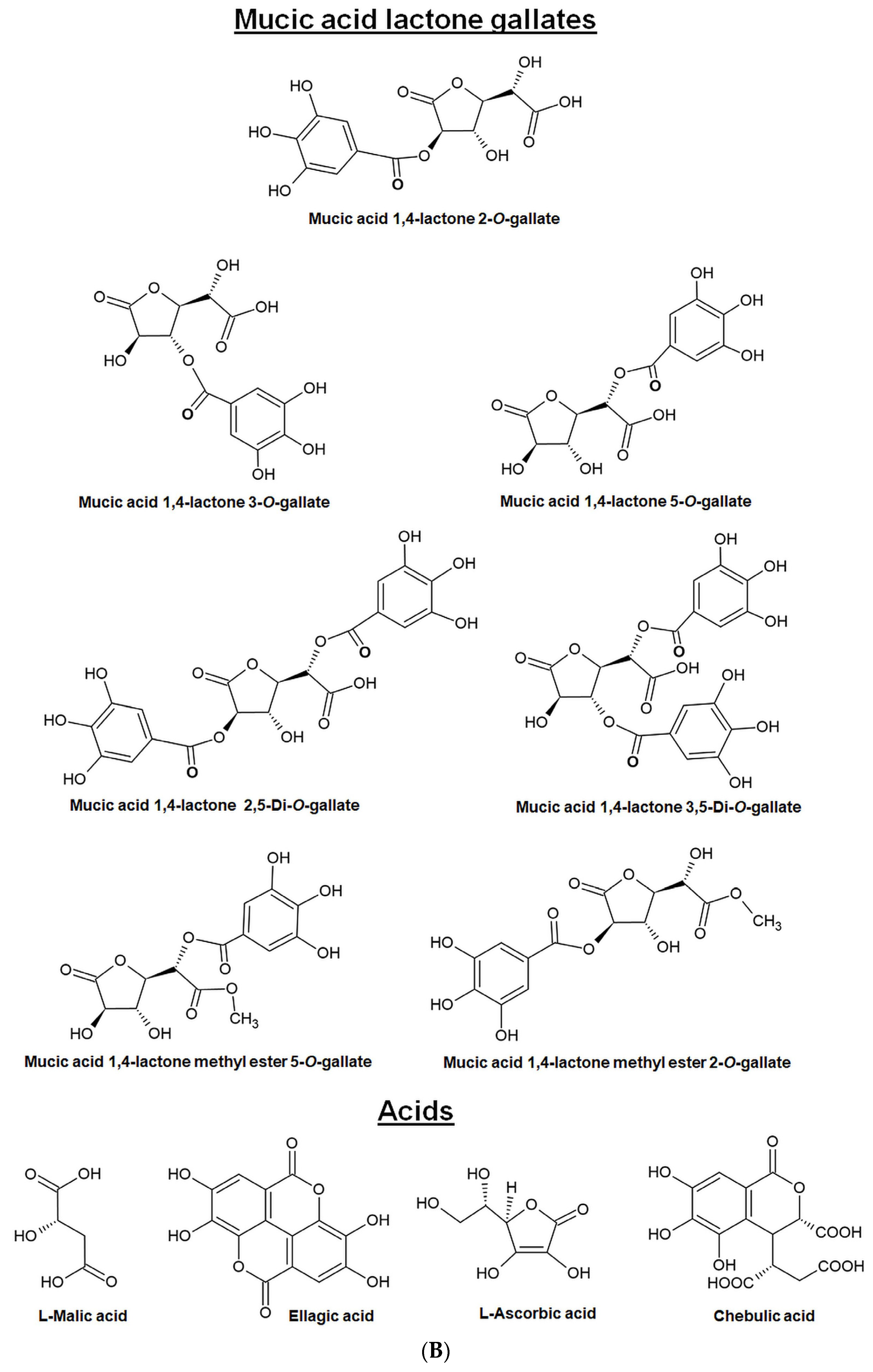
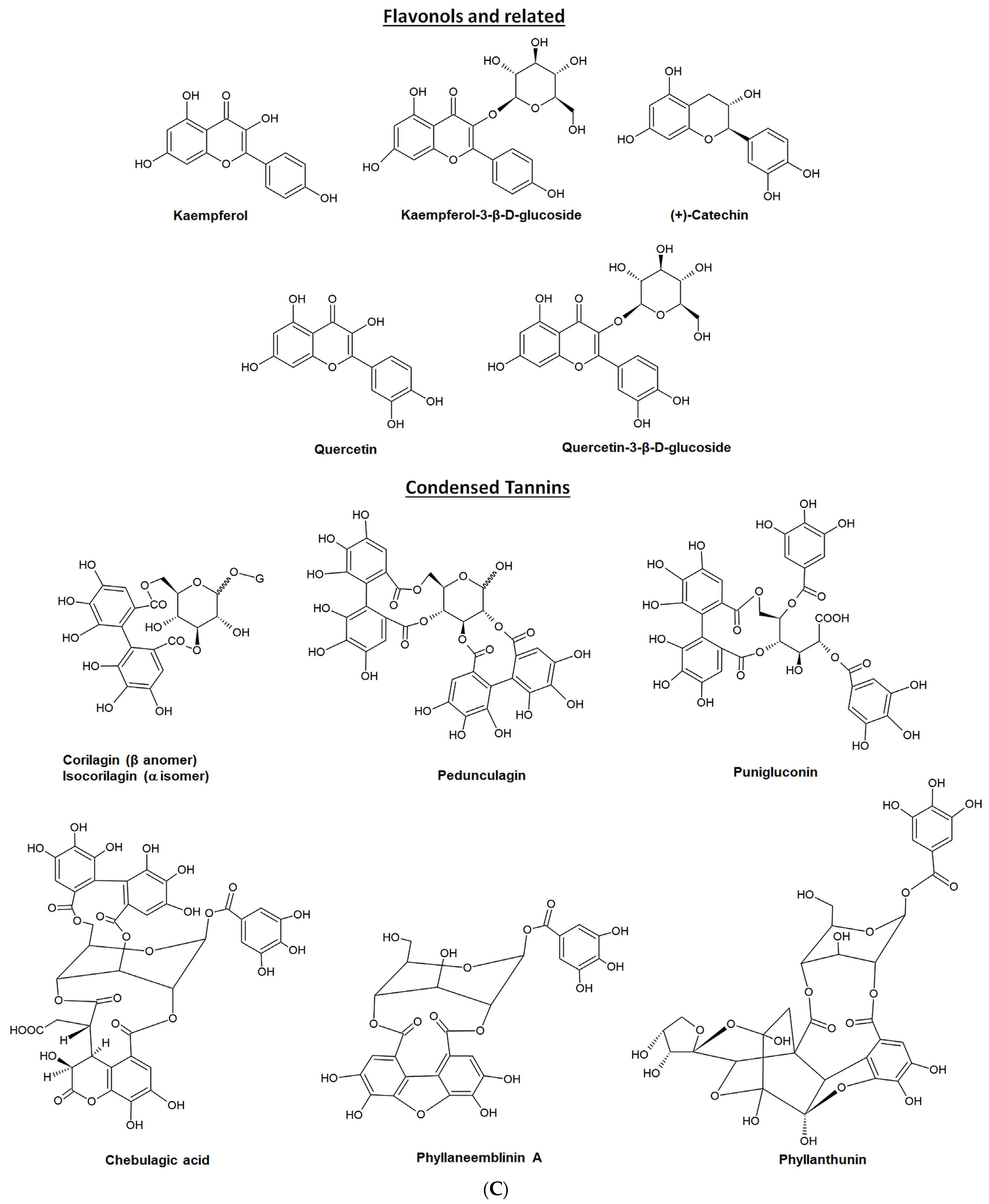
4. Major Phytochemicals (Bioactive Components) in EOF
5. Anti-Diabetic Activities of E. officinalis: Mechanism of Anti-Diabetic Action
5.1. Antioxidant Activities of EOF
5.2. Inhibition of PARP/PARG Activation by EOF
5.3. EOF Regulates Carbohydrate Metabolizing Enzymes
5.4. EOF Improves Glucose Homeostasis
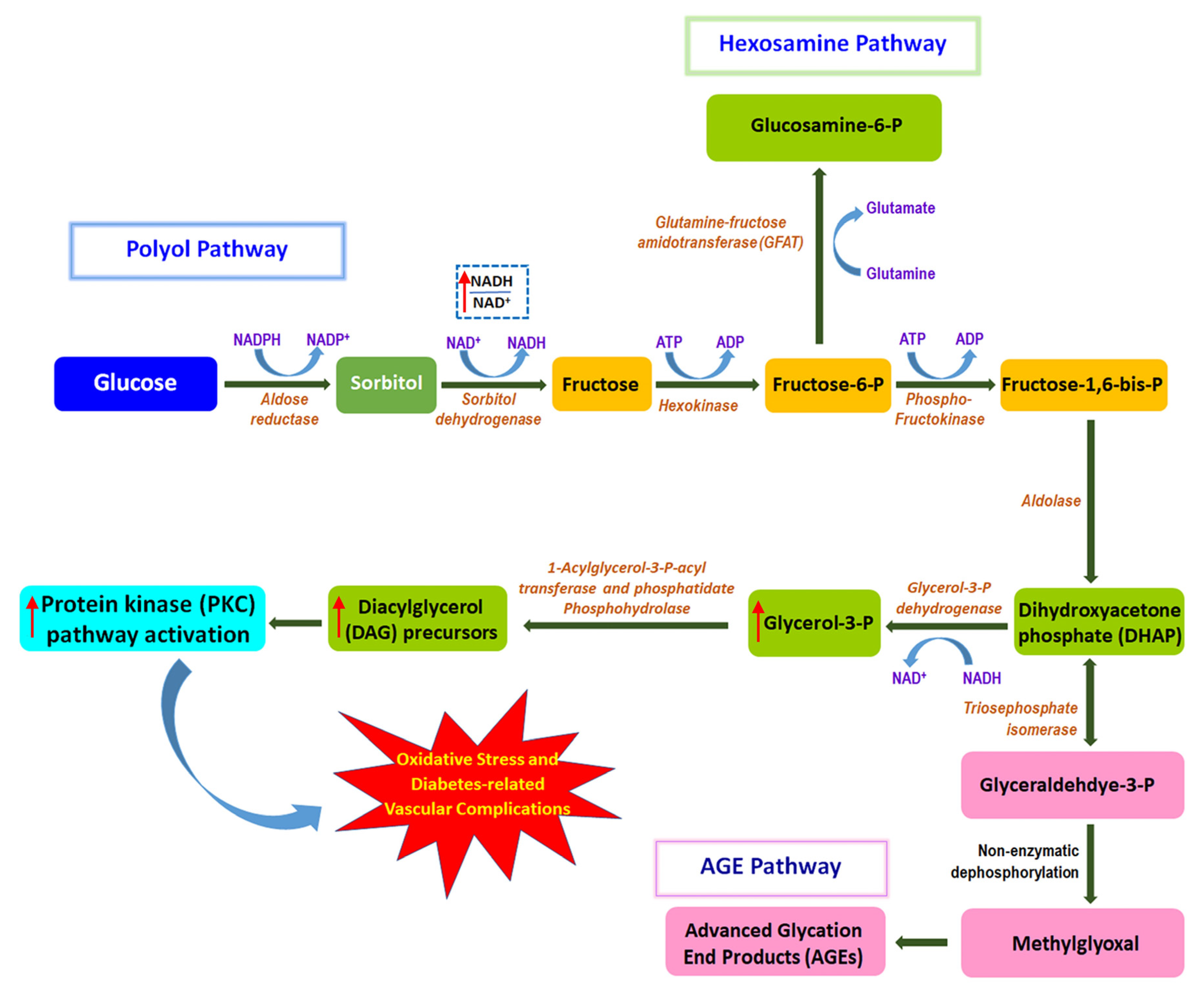
5.5. Inhibition of Polyol Pathway by EOF
5.6. EOF Alleviates Mitochondrial Dysfunction in Diabetes
5.7. E. officinalis Induces the Regeneration and Rejuvenation of Pancreatic β-Cells
5.8. EOF Reduces the Formation of AGEs
5.9. E. officinalis Modulates Diabetes-Induced Gut Dysbiosis
5.10. E. officinalis Modulates Cellular Energy Pathways
6. Evidence from Clinical Studies
| Experimental Model | EOF Supplement/Dosage and Duration | Study Outcome | References |
|---|---|---|---|
| STZ (200 mg/kg i.p.)-induced diabetic rats | EOF powder: 5, 10, and 15% diet for 21 days | Reduced blood glucose by 21–39% as compared to the basal value. | [92] |
| STZ (45 mg/kg)-induced diabetic rats | EOF-Fresh Juice: 5 mL/kg/day and hydroalcoholic extract 100 mg/kg/day | Significantly decreased the serum glucose level and increased the glucose tolerance. | [93] |
| Dexamethasone (10 mg/kg, i.p)-induced insulin resistance in diabetic rat models | EOF extract: 40, 60, and 80 mg/kg doses for 8 days | Showed a significant reduction in blood glucose level similar to Glimepiride (20 mg/kg bw). | [94] |
| Sucrose (2.5 g/kg body weight) treated Long Evans | EOF extract: 1.25 g/kg | Reduced the percentage of intestinal glucose absorption significantly in a time-dependent manner. | [95] |
| Beetal goat kids under mild heat stress | EOF extract: 2% of the diet for 90 days | Significantly reduced the blood glucose levels, total cholesterol (TC), and TG levels in the serum as compared to the non-supplemented group. | [96] |
| STZ (70 mg/kg, i.p.)-induced diabetic Wistar rats | Aqueous EOF extract: 200 and 400 mg/kg for 11 weeks | Significantly reduced blood glucose levels only at the higher dose (400 mg/kg) from week three to the end of the study (week 11). The effect was similar to metformin (600 mg/kg bw). | [43] |
| Neonatal STZ (90 mg/kg, i.p.)-induced diabetic rats | Methanolic EOF extract: 250 or 500 mg/kg for 28 days | Significantly decreased the fasting blood glucose (FBS) levels and increased serum insulin in a dose- and time-dependent manner comparable to chlorpropamide (5 mg/kg bw). | [97] |
| STZ (40 mg/kg bw)-induced diabetic Male Albino Wistar rats | Ethanolic EOF extract: 200 mg/kg bw for 45 days | Significantly reduced blood glucose and elevated plasma insulin levels similar to glibenclamide (600 μg/kg bw). | [98] |
| Alloxan (120 mg/kg)-induced male albino diabetic rats | Aqueous EOF extract: 200 mg/kg | Significantly decreased the blood glucose levels as well as TG in a time-dependent manner comparable to chlorpropamide (120 mg/kg). | [99] |
| Glucose-induced Wistar rats | Ethanolic EOF extract: 100 and 200 mg/kg for 14 days | Significantly reduced the blood glucose level similar to metformin (65 mg/kg). | [100] |
| Arsenic (3 mg/kg bw/day) induced mice with hyperglycemia | EOF extract: 500 mg/kg for 30 days | EOF balanced blood sugar level and hepatic glucose regulatory enzyme in arsenic-induced hyperglycemic mice. It also increased the serum insulin levels. | [83] |
7. E. officinalis against Diabetes-Induced Complications
7.1. Diabetic Retinopathy
7.2. Diabetic Neuropathy
7.3. Diabetic Nephropathy (DN)
7.4. Diabetic Hepatopathy
7.5. Diabetes-Induced Cardiac Complications
7.5.1. Cardiovascular Diseases
7.5.2. Endothelial Dysfunction
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregg, E.; Buckley, J.; Ali, M.; Davies, J.; Flood, D.; Griffiths, B.; Lim, L.; Manne-Goehler, J.; Pearson-Stuttard, J.; Shaw, J. Improving Health Outcomes of People with Diabetes Mellitus: Target Setting to Reduce the Global Burden of Diabetes Mellitus by 2030; World Health Organization: Geneva, Switzerland, 2021; pp. 1–26. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.L.; Kochanek, K.D.; Xu, J.; Arias, E. Mortality in the United States, 2020. NCHS Data Brief, No. 427. Available online: https://www.cdc.gov/nchs/products/databriefs/db427.htm (accessed on 29 March 2023).
- Ahmed, A.M. History of diabetes mellitus. Saudi Med. J. 2002, 23, 373–378. [Google Scholar]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Mehta, S.; Singh, R.K.; Jaiswal, D.; Rai, P.K.; Watal, G. Anti-diabetic activity of Emblica officinalis in animal models. Pharm. Biol. 2009, 47, 1050–1055. [Google Scholar] [CrossRef]
- Oh, K.K.; Adnan, M.; Cho, D.H. Network pharmacology of bioactives from Sorghum bicolor with targets related to diabetes mellitus. PLoS ONE 2020, 15, e0240873. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, H.U.; Adnan, M.; Oh, K.K.; Cho, D.H. Decrypting molecular mechanism insight of Phyllanthus emblica L. fruit in the treatment of type 2 diabetes mellitus by network pharmacology. Phytomed. Plus 2021, 1, 100144. [Google Scholar] [CrossRef]
- Huang, H.-Z.; Qiu, M.; Lin, J.-Z.; Li, M.-Q.; Ma, X.-T.; Ran, F.; Luo, C.-H.; Wei, X.-C.; Xu, R.-C.; Tan, P. Potential effect of tropical fruits Phyllanthus emblica L. for the prevention and management of type 2 diabetic complications: A systematic review of recent advances. Eur. J. Nutr. 2021, 60, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Rastogi, S.; Rawat, A. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid. Based Complement. Alternat. Med. 2013, 2013, 376327. [Google Scholar] [CrossRef]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar] [CrossRef]
- Yadav, S.S.; Singh, M.K.; Singh, P.K.; Kumar, V. Traditional knowledge to clinical trials: A review on therapeutic actions of Emblica officinalis. Biomed Pharm. 2017, 93, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- D’souza, J.J.; D’souza, P.P.; Fazal, F.; Kumar, A.; Bhat, H.P.; Baliga, M.S. Anti-diabetic effects of the Indian indigenous fruit Emblica officinalis Gaertn: Active constituents and modes of action. Food Funct. 2014, 5, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Ata, A.V.; Anil Kumar, N.; Sharopov, F.; Ramirez-Alarcon, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 2016, 111, 180–200. [Google Scholar] [CrossRef]
- Gul, M.; Liu, Z.-W.; Rabail, R.; Faheem, F.; Walayat, N.; Nawaz, A.; Shabbir, M.A.; Munekata, P.E.; Lorenzo, J.M.; Aadil, R.M. Functional and Nutraceutical Significance of Amla (Phyllanthus Emblica L.): A Review. Antioxidants 2022, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, P. Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica. J. Agric. Food Chem. 2014, 62, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Sood, V.; Colleran, K.; Burge, M.R. Thiazolidinediones: A comparative review of approved uses. Diabetes Technol. Ther. 2000, 2, 429–440. [Google Scholar] [CrossRef]
- Rojas, L.B.; Gomes, M.B. Metformin: An old but still the best treatment for type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Brunton, S.A.; Wysham, C.H. GLP-1 receptor agonists in the treatment of type 2 diabetes: Role and clinical experience to date. Postgrad. Med. 2020, 132, 3–14. [Google Scholar] [CrossRef]
- Ahrén, B. DPP-4 Inhibition and the Path to Clinical Proof. Front. Endocrinol. 2019, 10, 376. [Google Scholar] [CrossRef]
- Wright, E.M. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, S.; Kang, B.; Zhou, J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc. Diabetol. 2022, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Lopez Vicchi, F.; Luque, G.M.; Brie, B.; Nogueira, J.P.; Garcia Tornadu, I.; Becu-Villalobos, D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol. Res. 2016, 109, 74–80. [Google Scholar] [CrossRef]
- Michaud, L.; Seplowe, M.; Meir, J.; Aronow, W.S. Safety and cardiometabolic efficacy of novel antidiabetic drugs. Expert. Opin. Drug Saf. 2023, 22, 119–124. [Google Scholar] [CrossRef]
- Wu, M.; Cai, J.; Fang, Z.; Li, S.; Huang, Z.; Tang, Z.; Luo, Q.; Chen, H. The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit. Nutrients 2022, 14, 857. [Google Scholar] [CrossRef]
- Majeed, M.; Bhat, B.; Jadhav, A.N.; Srivastava, J.S.; Nagabhushanam, K. Ascorbic acid and tannins from Emblica officinalis Gaertn. Fruits—A revisit. J. Agric. Food Chem. 2009, 57, 220–225. [Google Scholar] [CrossRef]
- Olennikov, D.; Kashchenko, N.; Schwabl, H.; Vennos, C.; Loepfe, C. New mucic acid gallates from Phyllanthus emblica. Chem. Nat. Compd. 2015, 51, 666–670. [Google Scholar] [CrossRef]
- She, G.; Cheng, R.; Sha, L.; Xu, Y.; Shi, R.; Zhang, L.; Guo, Y. A novel phenolic compound from Phyllanthus emblica. Nat. Prod. Commun. 2013, 8, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-F.; Lv, Q.; Ji, X.; Fang, C.; Fei, J.-X.; Liu, X.-J.; Liu, J.-X.; Liu, X.-H. Three New Antioxidative Phenolics from Phyllanthus emblica L. Fruit. Nat. Prod. Commun. 2023, 18, 1934578X231155717. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, H.T.; Cheng, R.R.; Wang, D.; Yang, C.R.; Tanaka, T.; Kouno, I.; Zhang, Y.J. Antioxidant and hyaluronidase inhibitory activities of diverse phenolics in Phyllanthus emblica. Nat. Prod. Res. 2016, 30, 2726–2729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Miao, D.; Zhu, W.F.; Xu, J.; Liu, W.Y.; Kitdamrongtham, W.; Manosroi, J.; Abe, M.; Akihisa, T.; Feng, F. Biological Activities of Phenolics from the Fruits of Phyllanthus emblica L. (Euphorbiaceae). Chem. Biodivers. 2017, 14, e1700404. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Tanaka, T.; Yang, C.R.; Kouno, I. New phenolic constituents from the fruit juice of Phyllanthus emblica. Chem. Pharm. Bull. 2001, 49, 537–540. [Google Scholar] [CrossRef]
- Yang, C.B.; Zhang, F.; Deng, M.C.; He, G.Y.; Yue, J.M.; Lu, R.H. A New Ellagitannin from the Fruit of Phyllanthus emblica L. J. Chin. Chem. Soc. 2007, 54, 1615–1618. [Google Scholar] [CrossRef]
- Ghosal, S. Active constituents of Emblica officinalis: Part I. The chemistry and antioxidative effects of two new hydrolysable tannins, Emblicanin A and B. Indian J. Chem. 1996, 35, 941–948. [Google Scholar]
- Dasaroju, S.; Gottumukkala, K.M. Current trends in the research of Emblica officinalis (Amla): A pharmacological perspective. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 150–159. [Google Scholar]
- Majeed, M.; Majeed, S.; Mundkur, L.; Nagabhushanam, K.; Arumugam, S.; Beede, K.; Ali, F. Standardized Emblica officinalis fruit extract inhibited the activities of α-amylase, α-glucosidase, and dipeptidyl peptidase-4 and displayed antioxidant potential. J. Sci. Food. Agric. 2020, 100, 509–516. [Google Scholar] [CrossRef]
- Majeed, M.; Mundkur, L.; Paulose, S.; Nagabhushanam, K. Novel Emblica officinalis extract containing β-glucogallin vs. metformin: A randomized, open-label, comparative efficacy study in newly diagnosed type 2 diabetes mellitus patients with dyslipidemia. Food Funct. 2022, 13, 9523–9531. [Google Scholar] [CrossRef]
- Elobeid, M.A.; Ahmed, E.A. Antidiabetic efficacy of aqueous fruit extract of Amla (Emblica officinalis, Gaertn) in streptozotocin-induced diabetes mellitus in male rats. Trop. J. Pharm. Res. 2015, 14, 801–806. [Google Scholar] [CrossRef]
- Ansari, P.; Tabasumma, N.; Snigdha, N.N.; Siam, N.H.; Panduru, R.V.; Azam, S.; Hannan, J.; Abdel-Wahab, Y.H. Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology 2022, 3, 159–175. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Naik, G.; Priyadarsini, K.; Bhagirathi, R.; Mishra, B.; Mishra, K.; Banavalikar, M.; Mohan, H. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother. Res. 2005, 19, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Poltanov, E.A.; Shikov, A.N.; Dorman, H.D.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R. Chemical and antioxidant evaluation of Indian gooseberry (Emblica officinalis Gaertn., syn. Phyllanthus emblica L.) supplements. Phytother. Res. 2009, 23, 1309–1315. [Google Scholar] [CrossRef]
- Packirisamy, R.M.; Bobby, Z.; Panneerselvam, S.; Koshy, S.M.; Jacob, S.E. Metabolomic analysis and antioxidant effect of Amla (Emblica officinalis) extract in preventing oxidative stress-induced red cell damage and plasma protein alterations: An in vitro study. J. Med. Food 2018, 21, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandel, S.; Ghosh, A.; Matta, T.; Gautam, A.; Bhattacharya, A.; Babu, S.S.; Sukla, S.; Nag, D.; Ravichandiran, V.; et al. Glucogallin Attenuates the LPS-Induced Signaling in Macrophages and Protects Mice against Sepsis. Int. J. Mol. Sci. 2022, 23, 11254. [Google Scholar] [CrossRef]
- Puppala, M.; Ponder, J.; Suryanarayana, P.; Reddy, G.B.; Petrash, J.M.; LaBarbera, D.V. The Isolation and Characterization of β-Glucogallin as a Novel Aldose Reductase Inhibitor from Emblica officinalis. PLoS ONE 2012, 7, e31399. [Google Scholar] [CrossRef]
- Khan, A.N.; Singh, R.; Bhattacharya, A.; Kumar, S.; Ghosh, A.; Nag, D.; Ravichandiran, V.; Ghosh, D. Glucogallin Attenuates RAW 264.7 Cells from Arsenic Trioxide Induced Toxicity via the NF-ҡB/NLRP3 Pathway. Molecules 2022, 27, 5263. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ferdous, K.U.; Roy, S.; Nitul, I.A.; Mamun, F.; Hossain, M.H.; Subhan, N.; Alam, M.A.; Haque, M.A. Polyphenolic compounds of amla prevent oxidative stress and fibrosis in the kidney and heart of 2K1C rats. Food Sci. Nutr. 2020, 8, 3578–3589. [Google Scholar] [CrossRef]
- Rao, T.; Sakaguchi, N.; Juneja, L.; Wada, E.; Yokozawa, T. Amla (Emblica officinalis Gaertn.) extracts reduce oxidative stress in streptozotocin-induced diabetic rats. J. Med. Food 2005, 8, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Seghieri, G.; Di Simplicio, P.; Anichini, R.; Alviggi, L.; De Bellis, A.; Bennardini, F.; Franconi, F. Platelet antioxidant enzymes in insulin-dependent diabetes mellitus. Clin. Chim. Acta 2001, 309, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Pingali, U.; Muralidhar, N. Study of pharmacodynamic interaction of Phyllanthus emblica extract with clopidogrel and ecosprin in patients with type II diabetes mellitus. Phytomedicine 2014, 21, 579–585. [Google Scholar] [CrossRef]
- Szabó, C. Roles of poly (ADP-ribose) polymerase activation in the pathogenesis of diabetes mellitus and its complications. Pharmacol. Res. 2005, 52, 60–71. [Google Scholar] [CrossRef]
- He, H.F. Recognition of Gallotannins and the Physiological Activities: From Chemical View. Front. Nutr. 2022, 9, 888892. [Google Scholar] [CrossRef]
- Nottbohm, A.C.; Hergenrother, P.J. The promises and pitfalls of small-molecule inhibition of poly (ADP-ribose) glycohydrolase (PARG). In Drug Discovery Research: New Frontiers in the Post-Genomic Era; Huang, Z., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 163–185. [Google Scholar] [CrossRef]
- Chandak, P.G.; Gaikwad, A.B.; Tikoo, K. Gallotannin ameliorates the development of streptozotocin-induced diabetic nephropathy by preventing the activation of PARP. Phytother. Res. 2009, 23, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Formentini, L.; Arapistas, P.; Pittelli, M.; Jacomelli, M.; Pitozzi, V.; Menichetti, S.; Romani, A.; Giovannelli, L.; Moroni, F.; Chiarugi, A. Mono-galloyl glucose derivatives are potent poly (ADP-ribose) glycohydrolase (PARG) inhibitors and partially reduce PARP-1-dependent cell death. Br. J. Pharmacol. 2008, 155, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.-N.; Li, H.-H.; Tan, B.; Zhang, M.; Xiao, Z.-G.; Tian, X.-H.; Zhai, X.-T.; Liu, M.; Liu, Y.-X.; Wang, L.-P. Free and bound phenolic profiles of the bran from different rice varieties and their antioxidant activity and inhibitory effects on α-amylose and α-glucosidase. J. Cereal. Sci. 2018, 82, 206–212. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.G.; Venugopalan, V.V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef]
- Sapkota, B.K.; Khadayat, K.; Sharma, K.; Raut, B.K.; Aryal, D.; Thapa, B.B.; Parajuli, N. Phytochemical Analysis and Antioxidant and Antidiabetic Activities of Extracts from Bergenia ciliata, Mimosa pudica, and Phyllanthus emblica. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 4929824. [Google Scholar] [CrossRef]
- Kalekar, S.A.; Munshi, R.P.; Bhalerao, S.S.; Thatte, U.M. Insulin sensitizing effect of 3 Indian medicinal plants: An in vitro study. Indian J. Pharmacol. 2013, 45, 30–33. [Google Scholar] [CrossRef]
- Punithavathi, V.; Stanely Mainzen Prince, P.; Kumar, M.; Selvakumari, C. Protective effects of gallic acid on hepatic lipid peroxide metabolism, glycoprotein components and lipids in streptozotocin-induced type II diabetic wistar rats. J. Biochem. Mol. Toxicol. 2011, 25, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Punithavathi, V.R.; Prince, P.S.M.; Kumar, R.; Selvakumari, J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur. J. Pharmacol. 2011, 650, 465–471. [Google Scholar] [CrossRef]
- Li, S.; Shin, H.J.; Ding, E.L.; van Dam, R.M. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2009, 302, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Geetha, K.; Vijay, R.; Chidrawar, U. Polyol pathway: A review on a potential target for the prevention of diabetic complications. Int. J. Pharm. Sci. Invent. 2014, 2, 696–711. [Google Scholar]
- Guo, L.; Ma, F.; Wei, F.; Fanella, B.; Allen, D.K.; Wang, X. Cytosolic phosphorylating glyceraldehyde-3-phosphate dehydrogenases affect Arabidopsis cellular metabolism and promote seed oil accumulation. Plant Cell 2014, 26, 3023–3035. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharm. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012, 3, 87. [Google Scholar] [CrossRef]
- Suryanarayana, P.; Kumar, P.A.; Saraswat, M.; Petrash, J.M.; Reddy, G.B. Inhibition of aldose reductase by tannoid principles of Emblica officinalis: Implications for the prevention of sugar cataract. Mol. Vis. 2004, 10, 148–154. [Google Scholar]
- Suryanarayana, P.; Saraswat, M.; Petrash, J.M.; Reddy, G.B. Emblica officinalis and its enriched tannoids delay streptozotocin-induced diabetic cataract in rats. Mol. Vis. 2007, 13, 1291–1297. [Google Scholar] [PubMed]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (dys) function and insulin resistance: From pathophysiological molecular mechanisms to the impact of diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Yamamoto, H.; Morino, K.; Mengistu, L.; Ishibashi, T.; Kiriyama, K.; Ikami, T.; Maegawa, H. Amla enhances mitochondrial spare respiratory capacity by increasing mitochondrial biogenesis and antioxidant systems in a murine skeletal muscle cell line. Oxid. Med. Cell. Longev. 2016, 2016, 1735841. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.D.; Padmavathi, P.; Kavitha, G.; Gopi, S.; Varadacharyulu, N. Emblica officinalis ameliorates alcohol-induced brain mitochondrial dysfunction in rats. J. Med. Food 2011, 14, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Joly, E.; El-Assaad, W.; Roduit, R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: Role in β-cell adaptation and failure in the etiology of diabetes. Diabetes 2002, 51 (Suppl. 3), S405–S413. [Google Scholar] [CrossRef]
- Park, Y.J.; Woo, M. Pancreatic β cells: Gatekeepers of type 2 diabetes. J. Cell. Biol. 2019, 218, 1094–1095. [Google Scholar] [CrossRef]
- Sri, K.S.; Kasturi, K.; Sivannarayana, G. Impact of Pranayama and Amla, an approach towards the control of diabetes mellitus. Int. J. PharmTech Res. 2014, 6, 1157–1161. [Google Scholar]
- Sameermahmood, Z.; Raji, L.; Saravanan, T.; Vaidya, A.; Mohan, V.; Balasubramanyam, M. Gallic acid protects RINm5F β-cells from glucolipotoxicity by its antiapoptotic and insulin-secretagogue actions. Phytother. Res. 2010, 24, S83–S94. [Google Scholar] [CrossRef]
- Latha, R.; Daisy, P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2011, 189, 112–118. [Google Scholar] [CrossRef]
- Singh, M.K.; Dwivedi, S.; Yadav, S.S.; Yadav, R.S.; Khattri, S. Anti-diabetic Effect of Emblica-officinalis (Amla) against Arsenic Induced Metabolic Disorder in Mice. Indian J. Clin. Biochem. 2020, 35, 179–187. [Google Scholar] [CrossRef]
- Muthenna, P.; Akileshwari, C.; Reddy, G.B. Ellagic acid, a new antiglycating agent: Its inhibition of N ϵ-(carboxymethyl) lysine. Biochem. J. 2012, 442, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, S.Y.; Lin, J.A.; Yen, G.C. Preventive effect of Indian gooseberry (Phyllanthus emblica L.) fruit extract on cognitive decline in high-fat diet (HFD)-fed rats. Mol. Nutr. Food Res. 2023, 67, 2200791. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Brochot, A.; Azalbert, V.; Landrier, J.F.; Tourniaire, F.; Serino, M. A Two-Week Treatment with Plant Extracts Changes Gut Microbiota, Caecum Metabolome, and Markers of Lipid Metabolism in ob/ob Mice. Mol. Nutr. Food Res. 2019, 63, 1900403. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, Y.; Jiang, Y.; Wu, B.; Yu, Y. Aqueous Extract of Phyllanthus emblica L. Alleviates Functional Dyspepsia through Regulating Gastrointestinal Hormones and Gut Microbiome In Vivo. Foods 2022, 11, 1491. [Google Scholar] [CrossRef]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic potential of gallic acid from Emblica officinalis: Improved glucose transporters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine 2020, 73, 152906. [Google Scholar] [CrossRef]
- Khan, A.N.; Singh, R.; Bhattacharya, A.; Chakravarti, R.; Roy, S.; Ravichandiran, V.; Ghosh, D. A Short Review on Glucogallin and its Pharmacological Activities. Mini. Rev. Med. Chem. 2022, 22, 2820–2830. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Ramzan, A.; Ali, A.; Ahmad, M. Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int. J. Food Sci. Nutr. 2011, 62, 609–616. [Google Scholar] [CrossRef]
- Richa, S.; Disha, A. Effect of Emblica officinalis diet in streptozotocin diabetic mice. In Medicinal Plants: Phytochemistry, Pharmacology and Therapeutics; Gupta, V.K., Singh, G.D., Singh, S., Kaul, A., Eds.; Daya Publishing House: New Delhi, India, 2010; Volume 1, pp. 413–420. [Google Scholar]
- Tirgar, P.; Shah, K.; Patel, V.; Desai, T.; Goyal, R. Investigation into mechanism of action of anti-diabetic activity of Emblica officinalis on streptozotocin induced type I diabetic rat. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 672–682. [Google Scholar]
- Bashir, A.; Mushtaq, A.; Mehboob, T. Evaluation of antioxidant and Antidiabetic activity of Phyllanthus emblica (fruit). Biologia 2018, 64, 85–91. [Google Scholar]
- Sultana, Z.; Jami, S.I.; Ali, E.; Begum, M.; Haque, M. Investigation of antidiabetic effect of ethanolic extract of Phyllanthus emblica Linn. fruits in experimental animal models. Pharmacol. Pharm. 2014, 5, 11–18. [Google Scholar] [CrossRef]
- Madan, J.; Sindhu, S.; Gupta, M.; Poonia, J. Evaluation of Emblica officinalis and Mentha piperata supplementation on biochemical parameters in growing beetal kids. J. Cell Tissue Res. 2015, 15, 4811–4814. [Google Scholar]
- Fatima, N.; Hafizur, R.M.; Hameed, A.; Ahmed, S.; Nisar, M.; Kabir, N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur. J. Nutr. 2017, 56, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, M.; Mirunalini, S.; Karthishwaran, K.; Dhamodharan, G. Antidiabetic and antihyperlipidemic properties of Phyllanthus emblica Linn. (Euphorbiaceae) on streptozotocin induced diabetic rats. Pak. J. Nutr. 2010, 9, 43–51. [Google Scholar] [CrossRef]
- Qureshi, S.A.; Asad, W.; Sultana, V. The effect of Phyllantus emblica Linn on type-II diabetes, triglycerides and liver-specific enzyme. Pak. J. Nutr. 2009, 8, 125–128. [Google Scholar] [CrossRef]
- Musman, M.; Zakia, M.; Rahmayani, R.F.I.; Erlidawati, E.; Safrida, S. Pharmaceutical hit of anti type 2 Diabetes mellitus on the phenolic extract of Malaka (Phyllanthus emblica L.) flesh. Clin. Phytosci. 2019, 5, 47. [Google Scholar] [CrossRef]
- Gomathi, A.; Kamalam, S.; Jeevaanand, N. To Study the Efficacy of Amla and Okra Juice on Blood Glucose Level among Type 2 Diabetes in Selected Rural Settings of Puducherry. Community Public Health Nurs. 2020, 5, 81–85. [Google Scholar] [CrossRef]
- Iyer, U.; Joshi, A.; Dhruv, S. Impact of Amla (Embilica officinalis) supplementation on the glycemic and lipidemic status of type 2 diabetic subjects. J. Herb. Med. Toxicol. 2009, 3, 15–21. [Google Scholar]
- Sri, K.; Kumari, D.J.; Sivannarayana, G. Effect of Amla, an approach towards the control of diabetes mellitus. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 103–108. [Google Scholar]
- Walia, K.; Boolchandani, R.; Dhand, S.; Antony, B. Improving glycemic & lipidemic profile with amla powder (Emblica officinalis) supplementation in adults with type 2 diabetes mellitus. Int. J. Basic Appl. Med. Sci. 2015, 5, 251–258. [Google Scholar]
- Antony, B.; Merina, B.; Sheeba, V. AmlamaxTM in the management of dyslipidemia in humans. Indian J. Pharm. Sci. 2008, 70, 504. [Google Scholar] [CrossRef] [PubMed]
- Usharani, P.; Fatima, N.; Muralidhar, N. Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: A randomized, double-blind, controlled study. Diabetes Metab. Syndr. Obes. Targets Ther. 2013, 6, 275–284. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- King, G.L.; Brownlee, M. The cellular and molecular mechanisms of diabetic complications. Endocrinol. Metab. Clin. N. Am. 1996, 25, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Laffin, B.; Ponder, J.; Enzsöly, A.; Németh, J.; LaBarbera, D.V.; Petrash, J.M. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem. Biol. Interact. 2013, 202, 283–287. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, F.; Xu, Y. Protective Effect of β-Glucogallin on Damaged Cataract against Methylglyoxal Induced Oxidative Stress in Cultured Lens Epithelial Cells. Med. Sci. Monit. 2019, 25, 9310–9318. [Google Scholar] [CrossRef]
- Petrash, M. Natural compounds as lead therapeutic agents against diabetic eye disease. Acta Ophthalmol. 2012, 90, 2761. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef]
- Smith, S.; Normahani, P.; Lane, T.; Hohenschurz-Schmidt, D.; Oliver, N.; Davies, A.H. Prevention and Management Strategies for Diabetic Neuropathy. Life 2022, 12, 1185. [Google Scholar] [CrossRef]
- Tiwari, V.; Kuhad, A.; Chopra, K. Emblica officinalis corrects functional, biochemical and molecular deficits in experimental diabetic neuropathy by targeting the oxido-nitrosative stress mediated inflammatory cascade. Phytother. Res. 2011, 25, 1527–1536. [Google Scholar] [CrossRef]
- Kumar, N.P.; Annamalai, A.; Thakur, R. Antinociceptive property of Emblica officinalis Gaertn (Amla) in high fat diet-fed/low dose streptozotocin induced diabetic neuropathy in rats. Indian J. Exp. Biol. 2009, 47, 737–742. [Google Scholar]
- Lim, D.W.; Kim, J.G.; Kim, Y.T. Analgesic effect of Indian gooseberry (emblica officinalis fruit) extracts on postoperative and neuropathic pain in rats. Nutrients 2016, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Q.; Lai, S.; Yang, L.; Shi, G.; Wang, Q.; Luo, Z.; Zhao, R.; Yu, Y. Attenuation of diabetic nephropathy by Sanziguben Granule inhibiting EMT through Nrf2-mediated anti-oxidative effects in streptozotocin (STZ)-induced diabetic rats. J. Ethnopharmacol. 2017, 205, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J.; Alornyo, K.K.; Adams, I.; Atule, S.; Obeng-Kyeremeh, R.; Amoah, D.; Adjei, S. Targeting hepatic sulfane sulfur/hydrogen sulfide signaling pathway with α-lipoic acid to prevent diabetes-induced liver injury via upregulating hepatic CSE/3-MST expression. Diabetol. Metab. Syndr. 2022, 14, 148. [Google Scholar] [CrossRef]
- Cheng, P.C.; Hsu, S.R.; Cheng, Y.C. Association between Serum Albumin Concentration and Ketosis Risk in Hospitalized Individuals with Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 1269706. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Zaccardi, F.; Di Stasio, E.; Romitelli, F.; Santini, S.A.; Zuppi, C.; Ghirlanda, G. Oxidative stress, nitric oxide, and diabetes. Rev. Diabet. Stud. 2010, 7, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Goyal, R.K.; Shah, R.S.; Tirgar, P.R.; Jadav, P.D. Experimental study on effect of hydroalcoholic extract of Emblica officinalis fruits on glucose homeostasis and metabolic parameters. Ayu 2013, 34, 440–444. [Google Scholar] [CrossRef]
- Patel, S.S.; Shah, R.S.; Goyal, R.K. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian J. Exp. Biol. 2009, 47, 564–570. [Google Scholar]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Tung, Y.T.; Huang, C.Z.; Lin, J.H.; Yen, G.C. Effect of Phyllanthus emblica L. fruit on methionine and choline-deficiency diet-induced nonalcoholic steatohepatitis. J. Food Drug Anal. 2018, 26, 1245–1252. [Google Scholar] [CrossRef]
- Huang, D.W.; Chang, W.C.; Wu, J.S.; Shih, R.W.; Shen, S.C. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr. Res. 2016, 36, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.; Mitchell, G.; Pfeffer, M.; Neaton, J.D.; Norman, J.; Svendsen, K.; Grimm, R.; Cohen, J.; Stamler, J.; Group, M.R. Pulse pressure and cardiovascular disease–related mortality: Follow-up study of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 2002, 287, 2677–2683. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yokozawa, T.; Kim, H.Y.; Tohda, C.; Rao, T.P.; Juneja, L.R. Influence of amla (Emblica officinalis Gaertn.) on hypercholesterolemia and lipid peroxidation in cholesterol-fed rats. J. Nutr. Sci. Vitaminol. 2005, 51, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Kanthe, P.S.; Patil, B.S.; Bagali, S.C.; Reddy, R.C.; Aithala, M.R.; Das, K.K. Protective effects of ethanolic extract of Emblica officinalis (amla) on cardiovascular pathophysiology of rats, fed with high fat diet. J. Clin. Diagn. Res. 2017, 11, CC05–CC09. [Google Scholar] [CrossRef]
- Gidding, S.S.; Allen, N.B. Cholesterol and Atherosclerotic Cardiovascular Disease: A Lifelong Problem. J. Am. Heart Assoc. 2019, 8, e012924. [Google Scholar] [CrossRef]
- Antony, B.; Benny, M.; Kaimal, T. A Pilot clinical study to evaluate the effect of Emblica officinalis extract (Amlamax™) on markers of systemic inflammation and dyslipidemia. Indian J. Clin. Biochem. 2008, 23, 378–381. [Google Scholar] [CrossRef]
- Upadya, H.; Prabhu, S.; Prasad, A.; Subramanian, D.; Gupta, S.; Goel, A. A randomized, double blind, placebo controlled, multicenter clinical trial to assess the efficacy and safety of Emblica officinalis extract in patients with dyslipidemia. BMC Complement. Altern. Med. 2019, 19, 27. [Google Scholar] [CrossRef]
- Gopa, B.; Bhatt, J.; Hemavathi, K.G. A comparative clinical study of hypolipidemic efficacy of Amla (Emblica officinalis) with 3-hydroxy-3-methylglutaryl-coenzyme—A reductase inhibitor simvastatin. Indian J. Pharmacol. 2012, 44, 238–242. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D. Vascular complications in diabetes mellitus: The role of endothelial dysfunction. Clin. Sci. 2005, 109, 143–159. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Zheng, G.-H.; Qiu, Z. Emblic leafflower (Phyllanthus emblica L.) fruits ameliorate vascular smooth muscle cell dysfunction in hyperglycemia: An underlying mechanism involved in ellagitannin metabolite urolithin A. Evid. Based Complement. Alternat. Med. 2018, 2018, 8478943. [Google Scholar] [CrossRef]
- Nazarzadeh, M.; Bidel, Z.; Canoy, D.; Copland, E.; Wamil, M.; Majert, J.; Smith Byrne, K.; Sundström, J.; Teo, K.; Davis, B.R.; et al. Blood pressure lowering and risk of new-onset type 2 diabetes: An individual participant data meta-analysis. Lancet 2021, 398, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Goyal, R.K. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacogn. Res. 2011, 3, 239. [Google Scholar] [CrossRef]
- Bhatia, J.; Tabassum, F.; Sharma, A.K.; Bharti, S.; Golechha, M.; Joshi, S.; Sayeed Akhatar, M.; Srivastava, A.K.; Arya, D.S. Emblica officinalis exerts antihypertensive effect in a rat model of DOCA-salt-induced hypertension: Role of (p) eNOS, NO and oxidative stress. Cardiovasc. Toxicol. 2011, 11, 272–279. [Google Scholar] [CrossRef] [PubMed]

| Subjects | EOF Supplement/Dosage and Duration | Study Outcome | Reference |
|---|---|---|---|
| Newly diagnosed T2DM patients (n = 94) | Aqueous EOF concentrate containing 10% β-glucogallin and hydrolyzable tannins: 1 or 2 g/day for 90 days | Significantly decreased FBS and PPBS and hemoglobin A1c (HbA1c) dose-dependently comparable to metformin (500 mg) at 1 g dose; superior to metformin at 2 g dose. | [42] |
| T2DM patients (n = 10) | EOF Juice: 50 g/day for 3 months | FBS levels were significantly reduced in subjects on metformin (500 mg) + EOF juice better than the metformin treatment alone. | [101] |
| T2DM patients (n = 45) | Fresh EOF fruit: Approximately 35 g/day for 60 days. | Reduced the blood sugar values by around 37.9% compared to the baseline level. | [102] |
| T2DM subjects (n = 60) | Fresh EOF fruit: Approximately 35 g/day for 6 months | A significant decrease in FBS, PPBS and HbA1c and a significant increase in HDL were observed in the EOF-supplemented group. | [103] |
| T2DM patients (n = 32) | EOF powder: 1, 2, or 3 g/day for 21 days | The reduction in FBS and PPBS by E. officinalis fruit powder in diabetic patients is comparable to the effect of glibenclamide (5 mg). | [91] |
| T2DM patients (n = 60) | EOF powder: 10 g/day for 90 days | Significant reduction in the mean FBS, PPBS and HbA1c levels and a significant increase in the mean hemoglobin levels; improved the glycemic and lipidemic profile. These effects were comparable to those of metformin (500 mg) and glimepiride (1 mg). | [104] |
| T2DM patients (n = 30) | EOF Extract: 500 mg/day for 4 months | Improved all the lipid parameters; reduced total cholesterol, LDL cholesterol, and triglycerides; and enhanced HDL cholesterol. | [105] |
| T2DM patients (n = 30) | EOF Extract: 500 mg/twice daily for 12 weeks | Alleviated endothelial dysfunction by reducing the biomarkers of oxidative stress similar to atorvastatin (10 mg). | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, M.; Narayanan, N.K.; Mundkur, L.; Prakasan, P.; Nagabhushanam, K. Super Fruit Amla (Emblica officinalis, Gaertn) in Diabetes Management and Ensuing Complications: A Concise Review. Nutraceuticals 2023, 3, 329-352. https://doi.org/10.3390/nutraceuticals3030026
Majeed M, Narayanan NK, Mundkur L, Prakasan P, Nagabhushanam K. Super Fruit Amla (Emblica officinalis, Gaertn) in Diabetes Management and Ensuing Complications: A Concise Review. Nutraceuticals. 2023; 3(3):329-352. https://doi.org/10.3390/nutraceuticals3030026
Chicago/Turabian StyleMajeed, Muhammed, Narayanan K. Narayanan, Lakshmi Mundkur, Priji Prakasan, and Kalyanam Nagabhushanam. 2023. "Super Fruit Amla (Emblica officinalis, Gaertn) in Diabetes Management and Ensuing Complications: A Concise Review" Nutraceuticals 3, no. 3: 329-352. https://doi.org/10.3390/nutraceuticals3030026
APA StyleMajeed, M., Narayanan, N. K., Mundkur, L., Prakasan, P., & Nagabhushanam, K. (2023). Super Fruit Amla (Emblica officinalis, Gaertn) in Diabetes Management and Ensuing Complications: A Concise Review. Nutraceuticals, 3(3), 329-352. https://doi.org/10.3390/nutraceuticals3030026







