Abstract
Eccentric exercise induces muscle damage and inflammation, resulting in a state of reduced physical activity with muscle dysfunction and a feeling of tiredness after exercise. Creatine is known to act as an energy buffer, but it has also been suggested to exert inhibitory effects on muscle damage and peripheral inflammation. The purpose of this study was to test the hypothesis that creatine supplementation alleviates fatigue after eccentric exercise and to explore the mechanism of this effect. C57BL/6J mice were fed an AIN-93G-formulated control diet or a creatine-containing diet for 6 days and were then subjected to downhill running, a model of eccentric exercise, to assess the effects on the total creatine concentrations in skeletal muscle and brain tissue, spontaneous activity, the urine concentration of titin N-fragment, and inflammatory gene expression. The results showed that creatine supplementation significantly increased the total creatine concentrations in skeletal muscle and brain tissue. Furthermore, spontaneous activity significantly decreased after downhill running and creatine supplementation maintained a significantly higher level of spontaneous activity. In addition, creatine supplementation significantly suppressed the downhill-running-induced increase in the mRNA expression of genes encoding ICAM-1, E-selectin, CD18, and BKB1R in the soleus muscle and IL-1β in the hypothalamus. On the other hand, creatine supplementation did not clearly influence the urine concentration of titin N-fragment. These results indicate that creatine supplementation may alleviate fatigue after eccentric exercise by partially suppressing inflammation in slow-twitch skeletal muscle and brain tissue.
1. Introduction
Eccentric exercise is observed in a wide range of physical activities. It is required in sports involving situations such as changing direction, stopping, and descending [1]. Furthermore, eccentric exercise is utilized by the elderly and patients as a means of rehabilitation training that is less stressful on the circulatory and respiratory systems [2]. However, because of muscle damage and inflammation, it can easily lead to a state of reduced physical activity with muscle dysfunction and a feeling of tiredness, resulting in deteriorated exercise performance and quality of life, even after exercise [1,2]. In this study, we defined fatigue as a state of reduced physical activity after exercise. Although some interventions have been investigated as methods to reduce fatigue after eccentric exercise, an easy-to-use method without adverse effects is needed to address this issue [3,4,5].
Muscle damage is one of the most important factors of muscle dysfunction following eccentric exercise. When muscle damage occurs, the myocyte membrane is scarred and myofibrillar proteins are degraded [2,6]. Inflammation is also considered to be a cause of muscle dysfunction. Increased levels of inflammatory cytokine after acute eccentric exercise have been shown to cause insulin resistance, resulting in the delayed recovery of muscle glycogen storage, which is essential for endurance performance [7,8,9,10,11,12]. In addition, a study using knockout mice for the gene coding CD18, a molecule on the surface of leukocytes that is responsible for tissue infiltration, showed that eccentric-exercise-induced leukocyte infiltration into skeletal muscle may delay the recovery of muscle strength [13]. These findings suggest that not only inflammatory cytokines, but also leukocytes per se are a cause of muscle dysfunction after acute eccentric exercise.
After acute eccentric exercise, feelings of tiredness can dampen motivation for further activity, leading to reduced spontaneous activity, i.e., fatigue [14,15,16,17]. Therefore, we postulated that the mitigation of such feelings would contribute to alleviating fatigue. Although physiotherapy and dietary interventions have been used to reduce muscle dysfunction [3,4,5], more effective coping strategies could be developed by aiming to reduce the feelings of tiredness. Inflammatory cytokines in brain tissue are considered to be an important factor in feelings of tiredness [18]. Carmichael et al. reported that, after an acute eccentric exercise, brain IL-1β expression increased and spontaneous activity decreased and that the intracerebroventricular administration of an IL-1 receptor antagonist suppressed the decrease in physical activity [19,20]. Although other cytokines may be involved, IL-1β has been suggested to be a key factor in feelings of tiredness in various studies [21].
Creatine is a non-proteinogenic amino acid that is supplied to tissues through diet and biosynthesis [22]. Creatine is mostly localized in skeletal muscle and brain tissue, where it binds to phosphate to form phosphocreatine (PCr) [22,23]. Among the energy-producing systems, the ATP-PCr system is responsible for the most rapid form of energy production [24]. Creatine supplementation has been shown to increase the PCr concentration in skeletal muscle and to improve high-intensity exercise performance, promoting greater adaptation to training [22]. In addition, although creatine has been on the market for more than 25 years, minimal adverse events have been reported [22,25]. Creatine is now popular as a safe and effective supplement, especially for athletes seeking explosive power and/or muscle hypertrophy [22,24].
Creatine also exerts actions other than energy production. For example, the prior intravenous administration of PCr reportedly prevented cardiomyocyte damage caused by cardiac ischemia [26]. The effect of creatine on skeletal muscle damage has also been investigated by evaluating plasma creatine kinase activity and/or the lactate dehydrogenase level. Although significant inhibitory effects have been observed in some human studies, a recent meta-analysis did not provide a clear conclusion [27]. In addition, even though previous studies examining the effects of creatine supplementation on systemic inflammatory mediators after an acute exercise have yielded mixed results [28], accumulating evidence suggests that creatine has anti-inflammatory properties in peripheral tissues. In pulmonary vascular endothelial cells, creatine inhibited endothelial hyperpermeability and leukocyte adhesion induced by inflammatory cytokines [29]. Creatine also reportedly mitigated edema formation induced by the subcutaneous administration of carrageenan in mice [30]. Taking into consideration reports that orally ingested creatine can cross the blood–brain barrier [31,32] and that creatine supplementation assisted recovery from central nervous system disorders such as concussion and depression [33,34], creatine can likely exert an anti-inflammatory action directly in the brain. Therefore, we thought it would be a promising inquiry to evaluate the anti-inflammatory effects of creatine supplementation in peripheral and brain tissue after acute exercise.
However, the effects of creatine supplementation on myofibrillar damage, inflammation in skeletal muscle and brain tissue, and reduction in physical activity after eccentric exercise have not been comprehensively reported. Therefore, the purpose of this study was to test the hypothesis that creatine supplementation can alleviate fatigue after eccentric exercise through its suppressive effects on myofibrillar damage and/or inflammation.
2. Materials and Methods
2.1. Animals
Six-week-old male C57BL/6J mice were purchased (The Jackson Laboratory Japan, Kanagawa, Japan). Each mouse was kept individually in a cage with Care-feeaz (HAMRI, Ibaraki, Japan) at a temperature of 23 °C ± 3 °C and a relative humidity of 50% ± 20% under a 12-h light–dark cycle. Water and food were provided ad libitum. The animals were acclimated for at least 3 days after receipt. All animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee at Taisho Pharmaceutical Co., Ltd. (Saitama, Japan, approval number: AN13636-Z00, AN13862-Z01, AN13976-Z00, AN14033-Z00).
2.2. Exercise Protocol
A total of two training sessions were performed 3 days prior to the downhill running. During the training sessions, mice were forced to run on a motorized treadmill (PanLab, Barcelona, Spain) with a +8° inclination. Based on a report that uphill running causes less muscle damage than level or downhill running [35], we aimed to minimize the repeated bout effect and to examine the pure impact of an acute downhill running session by using uphill running during the training sessions. In the first training session, mice ran at 6 m/min for 2 min, 8 m/min for 2 min, 10 m/min for 3 min, and 12 m/min for 3 min. In the second training session, mice ran at 12 m/min for 2 min, 16 m/min for 2 min, 20 m/min for 3 min, and 22 m/min for 3 min.
During the downhill running session, mice were forced to run on the treadmill with a −8° inclination immediately after switching to the light phase of the light–dark cycle. Mice ran at a gradually increasing speed (starting at 16 m/min and increasing by 0.3 m/min every 1 min) for 20 min and then at 22 m/min for 100 min or more. Mice that were unable to run at 22 m/min for 100 min during the downhill running were excluded from the analysis.
2.3. Dietary Intervention
As shown in Table 1, the AIN-93G composition was used for the control diet, while the creatine diet containing 5% creatine monohydrate by diet weight (creatine monohydrate dose: approximately 120 mg per day) was made by replacing the cellulose with creatine monohydrate (Sigma-Aldrich, St. Louis, MO, USA) in AIN-93G. During the acclimation period, the animals were fed the control diet. From 6 days prior to the downhill running, they were fed only one of the diets until the end of the experiment. These diets were obtained from Oriental Yeast (Tokyo, Japan).

Table 1.
Composition of the diets.
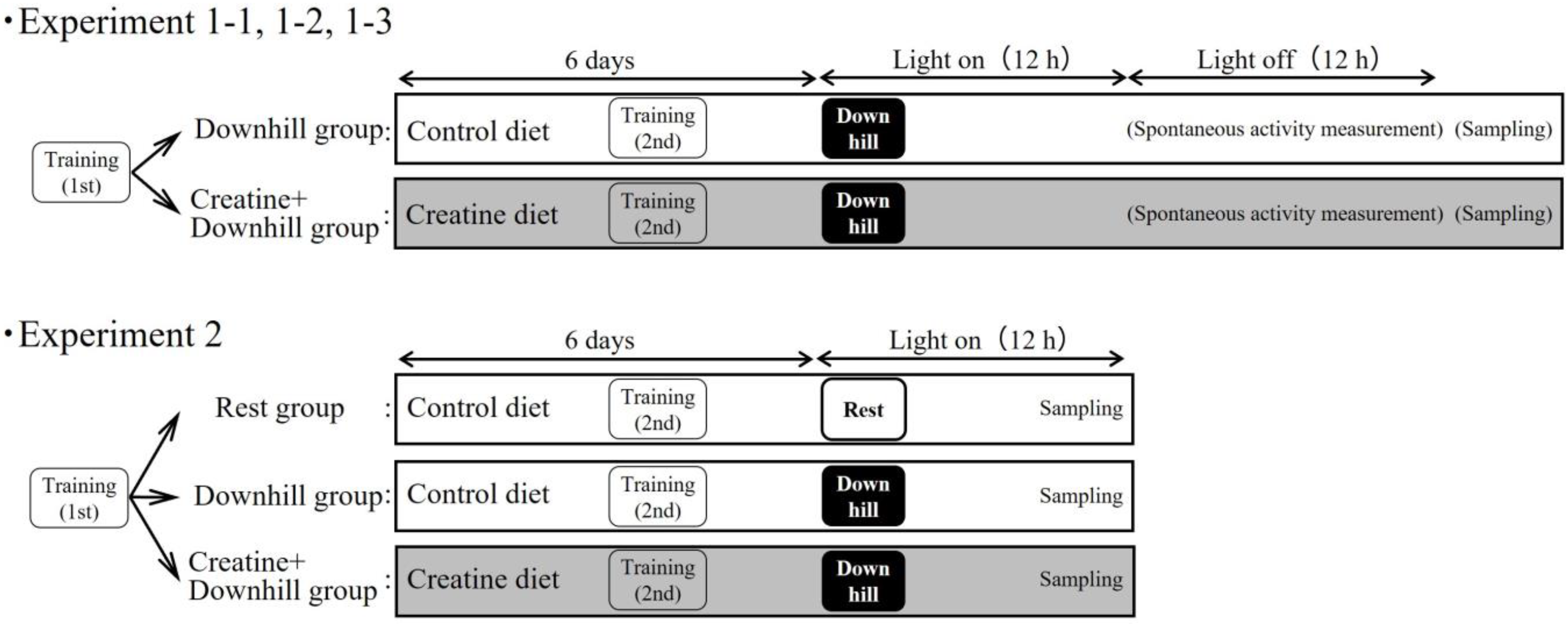
2.4. Experimental Design
An overview of the experiments is shown in Figure 1. To minimize the influence of the sampling itself on the indices, multiple experiments were conducted separately.
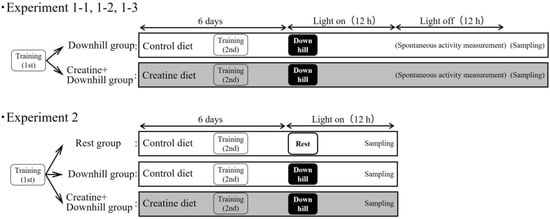
Figure 1.
Experiment design.
2.4.1. Experiment 1-1
Immediately after the first training session, 20 mice were randomly divided into two groups based on body weight and food intake during the acclimation period: a Downhill group (10 mice) that received the control diet, and a Creatine + Downhill group (10 mice) that received the creatine diet.
Six days after the grouping, the mice were forced to perform the downhill running and were then returned to each home-cage immediately thereafter. Approximately 24 h after the downhill running, the mice were euthanized under isoflurane anesthesia. Then, the soleus and plantaris muscles were removed. The samples were quickly frozen in liquid nitrogen and stored at −80 °C until the total creatine concentration measurements.
2.4.2. Experiment 1-2
Immediately after the first training session, 12 mice were randomly divided into 2 groups based on body weight and food intake during the acclimation period: a Downhill group (6 mice) that received the control diet, and a Creatine + Downhill group (6 mice) that received the creatine diet.
The procedure from downhill running to euthanasia was the same as that in experiment 1-1. Immediately after the euthanasia, the hypothalamus and cortex were removed. The samples were quickly frozen in liquid nitrogen and stored at −80 °C until the total creatine concentration measurement.
2.4.3. Experiment 1-3
Immediately after the first training session, 30 mice were randomly divided into two groups based on body weight and food intake during the acclimation period: a Downhill group (15 mice) that received the control diet, and a Creatine + Downhill group (15 mice) that received the creatine diet. Six days after the grouping, the mice were forced to perform the downhill running and were then returned to each home-cage immediately thereafter.
Spontaneous activity was measured using an infrared cage top motion sensor (SUPERMEX, Muromachi Kikai, Tokyo, Japan) installed in each home-cage. Since spontaneous activity is rarely observed during the light phase of the light–dark cycle, only the activity during the dark phase was used for the data calculation in this study. The average spontaneous activity during the 3 days before downhill running was used as the baseline value. The respective total spontaneous activity one day before (Pre) and on the day of (Post) the downhill running were expressed relatively to the baseline value for each mouse.
Two timepoints for urine collection were set at approximately 96 h before (Pre) and 24 h after (Post) the downhill running. Urine was discharged by stimulating the sacrum and was collected with a pipette. The urine samples were stored at −80 °C until the titin N-fragment measurement. Since 6 µL of urine sample was required for the measurement, mice that failed to provide a 6 µL urine sample at Pre or Post were excluded from the analysis.
2.4.4. Experiment 2
Immediately after the first training session, 42 mice were randomly divided into three groups based on body weight and food intake during the acclimation period: a Rest group (12 mice) that received the control diet without downhill running, a Downhill group (15 mice) that received the control diet with downhill running, and a Creatine + Downhill group (15 mice) that received the creatine diet with downhill running.
Six days after the grouping, mice other than those in the Rest group were forced to perform the downhill running and were then returned to each home-cage immediately thereafter. Approximately 8 h after the downhill running (immediately before the dark phase), the mice were euthanized under isoflurane anesthesia. Then, the soleus muscle, plantaris muscle, hypothalamus, and cortex were removed. The removed samples were quickly frozen in liquid nitrogen and stored at −80 °C until RNA extraction.
2.5. Measurement of Total Creatine Concentration in Skeletal Muscle and Brain Tissue
After the wet weight of each tissue was measured, the tissue was homogenized using a disposable homogenizer (PowerMasher II, Nippi, Tokyo, Japan). The Creatine Assay Kit (Sigma-Aldrich, St. Louis, MO, USA) was used following the protocol as instructed by the manufacturer to measure the amount of total creatine content in the homogenate. The total creatine concentration was calculated by dividing the total creatine content by the wet weight of each tissue.
2.6. Measurement of Urine Concentration of Titin N-Fragment
The amount of titin N-fragment in the urine sample was determined using the Mouse/Rat Titin N-Fragment Assay Kit (Immuno-Biological Laboratories, Gunma, Japan). The Protein Assay BCA Kit (Nacalai Tesque, Kyoto, Japan) was used to measure the amount of total protein in the sample. These methods were in accordance with the instructions of each kit. The amount of titin N-fragment was divided by the amount of total protein in each urine sample to calculate the urine concentration of titin N-fragment.
Titin is a myofibrillar protein and is considered to exert tension during the eccentric phase of exercise [36]. When it is degraded following tissue injury, its N-terminal fragment enters the urine [37]. The urine concentration of titin N-fragment has been utilized as an indicator of myofibrillar damage [38,39]. The advantage of this indicator is its low invasiveness. No puncture wound is necessary, thereby avoiding any effect on spontaneous activity.
2.7. RNA Extraction and Quantitative Real Time PCR
The frozen samples were immersed in RNAlater-ICE Frozen Tissue Transition Solution (Thermo Fisher Scientific, Waltham, MA, USA) and cooled to −20 °C for at least 16 h to stabilize the RNA in the samples. Each sample was then homogenized with a beads kit on a Precellys 24 tissue homogenizer (Bertin Technologies, Pas du Lac, France). RNA was then extracted using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Venlo, The Netherlands) for the muscle samples and the RNeasy Lipid Tissue Mini Kit (Qiagen, Venlo, The Netherlands) for the brain samples according to the manufacturers’ instructions. Based on the total RNA concentration measured with the NanoDrop 2000c (Thermo Fisher Scientific, Waltham, MA, USA), the concentrations between samples were aligned identically by adding Milli-Q water as appropriate.
cDNA was obtained by a reverse transcription reaction using ReverTra Ace® qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan). The cDNA was used as template, and quantitative real-time PCR was performed using THUNDERBIRD® SYBR qPCR Mix (TOYOBO, Osaka, Japan) on a StepOnePlus Real-time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The analysis was performed according to the standard curve method, using GAPDH as an internal standard for skeletal muscle and RPS18 as an internal standard for brain tissue. The mRNA expression level of gene in each sample was calculated relative to that of the internal standard, and that of each group was expressed relative to the mean value of that in the Rest group. The primer sequences (Takara Bio, Shiga, Japan) for the mRNAs of the genes coding each protein were as follows: tumor necrosis factor α (TNFα) (GeneBank accession number: NM_001278601.1): forward, GCCTCTTCTCATTCCTGCTTGTG and reverse, TGATGAGAGGGAGGCCATTTG; interleukin 1 beta (IL-1β) (GeneBank accession number: NM_008361.4): forward, CCAGGATGAGGACATGAGCAC and reverse, TGTTGTTCATCTCGGAGCCTGTA; interleukin 6 (IL-6) (GeneBank accession number: NM_001314054.1): forward, CCACTTCACAAGTCGGAGGCTTA and reverse, TGCAAGTGCATCATCGTTGTTC; intercellular adhesion molecule 1 (ICAM-1) (GeneBank accession number: NM_010493.3): forward, AACTGTGGCACCGTGCAGTC and reverse, AGGGTGAGGTCCTTGCCTACTTG; E-selectin (GeneBank accession number: NM_011345.2): forward, GATGCCGCCTACCTGTGAA and reverse, AGCAATGAGGACGATGTCAGGA; cluster of differentiation 18 (CD18) (GeneBank accession number: NM_008404.5): forward, CGAGTGTGACAATGTCAACTGTGAG and reverse, CGTAACCGGGCTTGCAACTA; bradykinin beta 1 receptor (BKB1R) (GeneBank accession number: NM_007539.3): forward, ACCTGCCTGCTCATCTGGGTA and reverse, GGATGCAGGCAGAGATGTTCA; bradykinin beta 2 receptor (BKB2R) (GeneBank accession number: NM_009747.2): forward, AGAACCCGTCCAGATGGAGA and reverse, CAATCCTCACACACTTGGCAGTA; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (GeneBank accession number: NM_008084.3): forward, TGTGTCCGTCGTGGATCTGA and reverse, TTGCTGTTGAAGTCGCAGGAG; ribosomal protein S18 (RPS18) (GeneBank accession number: NM_011296.3): forward, TTCTGGCCAACGGTCTAGACAAC and reverse, CCAGTGGTCTTGGTGTGCTGA.
2.8. Statistical Analyses
Calculations were conducted using Microsoft Excel 2016 (Microsoft, Redmond, WA, USA), and data are shown as the means ± SEM. Statistical analyses were performed using SAS 9.4 (EPS, Tokyo, Japan). The normality of the data was examined using the Shapiro–Wilk test. A p value < 0.05 was considered statistically significant.
For the total creatine concentrations, food intakes and body weights in experiment 1-1, 1-2, and 1-3, comparisons were made between two groups using the Student t-test (data with normality and homoscedasticity) or the Wilcoxon rank sum test (data without normality or homoscedasticity). The homogeneity of variance was confirmed using the F-test. For spontaneous activity and the urine concentration of titin N-fragment in experiment 1-3, analyses were conducted using a 2 × 2 (diet × time) repeated-measurement ANOVA.
In experiment 2, multiple comparisons were performed for mRNA expression levels, body weights, and food intakes. The Tukey–Kramer test was used for the data with normality and homoscedasticity, while the Steel–Dwass test was used for the data without normality or homoscedasticity. The homogeneity of variance was examined using the Bartlett test.
3. Results
3.1. Experiment 1-1
Eight mice in the Downhill group and seven mice in the Creatine + Downhill group were able to run downhill for 100 min or more (for 120 min at 22 m/min in all mice). After grouping, the food intake of each group was 2.4 ± 0.1 g/day in the Downhill group and 2.5 ± 0.1 g/day in the Creatine + Downhill group. The body weight just before the downhill running was 21.0 ± 0.4 g in the Downhill group and 20.7 ± 0.4 g in the Creatine + Downhill group. No significant differences in these indices were seen between the two groups.
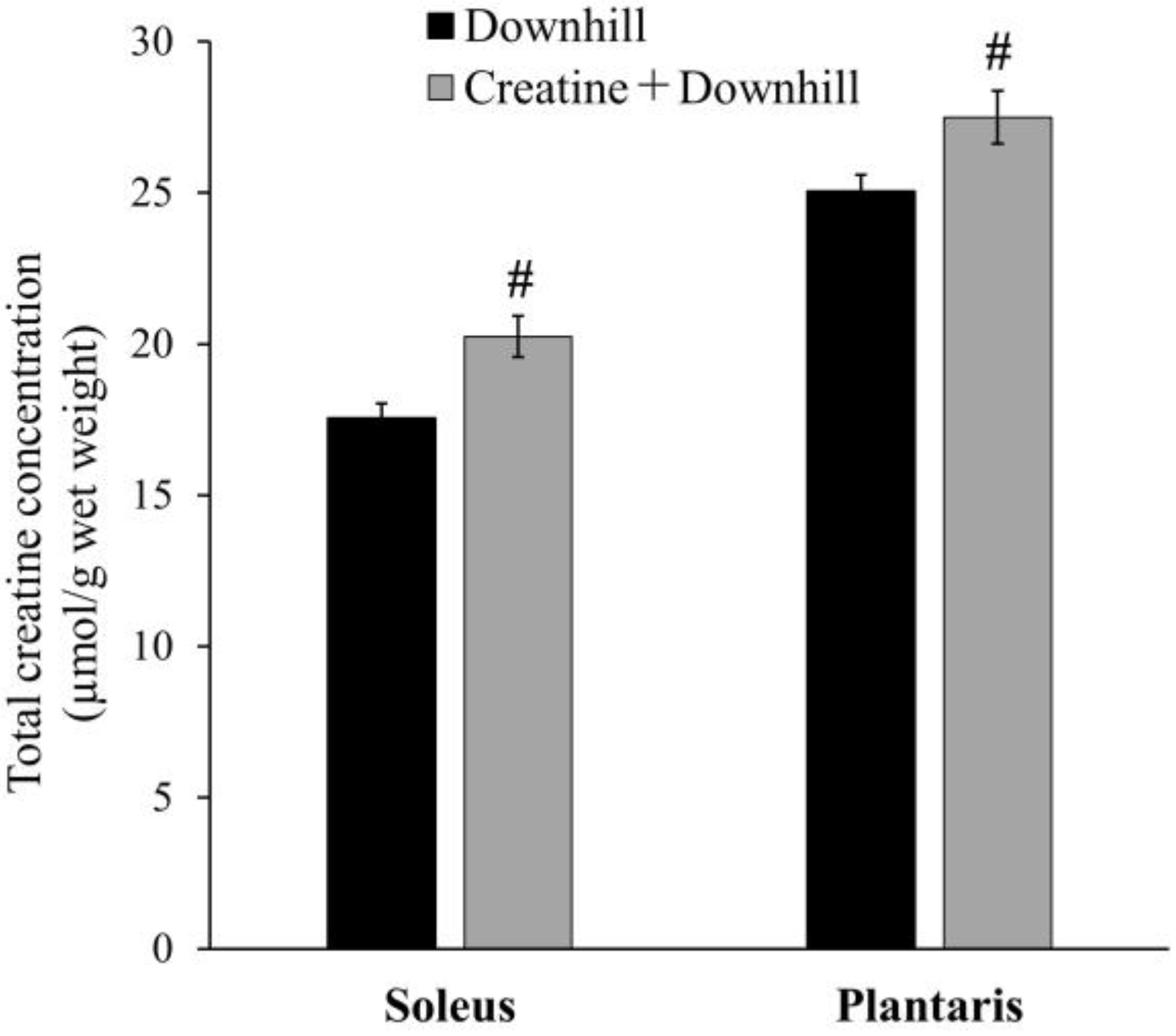
The total creatine concentration in skeletal muscle is shown in Figure 2. In the soleus and plantaris muscles, the Creatine + Downhill group had significantly higher values than the Downhill group (15.4% ± 3.9% and 9.7% ± 3.5%, respectively). Since the extent of this increase was comparable to that observed in human studies with creatine supplementation [22,40], the dietary intervention protocol in this study was deemed to be physiologically relevant.
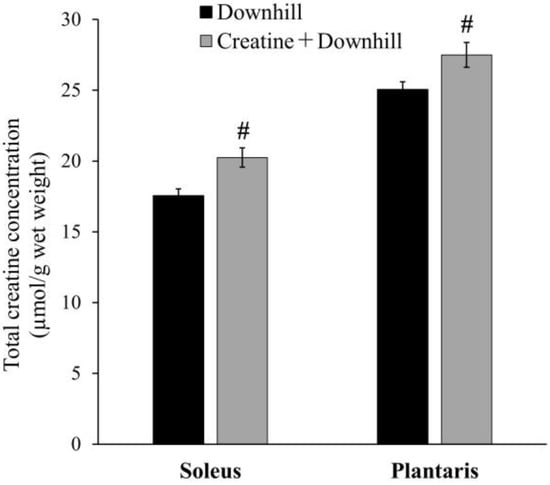
Figure 2.
Total creatine concentration in skeletal muscle of the Downhill group (black bar, n = 8) and the Creatine + Downhill group (gray bar, n = 7). Values are the means ± SEM. # indicates a significant difference from the Downhill group in the equivalent muscle (p < 0.05).
3.2. Experiment 1-2
Six mice in the Downhill group and six mice in the Creatine + Downhill group were able to run downhill for 100 min or more. The running time ranged from 100 to 160 min, with a mean ± SEM of 121.0 ± 12.3 min in the Downhill group and 150.0 ± 6.5 min in the Creatine + Downhill group. After grouping, the food intake of each group was 2.3 ± 0.1 g/day in the Downhill group and 2.4 ± 0.1 g/day in the Creatine + Downhill group. The body weight just before the downhill running was 21.6 ± 0.3 g in the Downhill group and 21.6 ± 0.3 g in the Creatine + Downhill group. No significant differences in these indices were seen between the two groups.
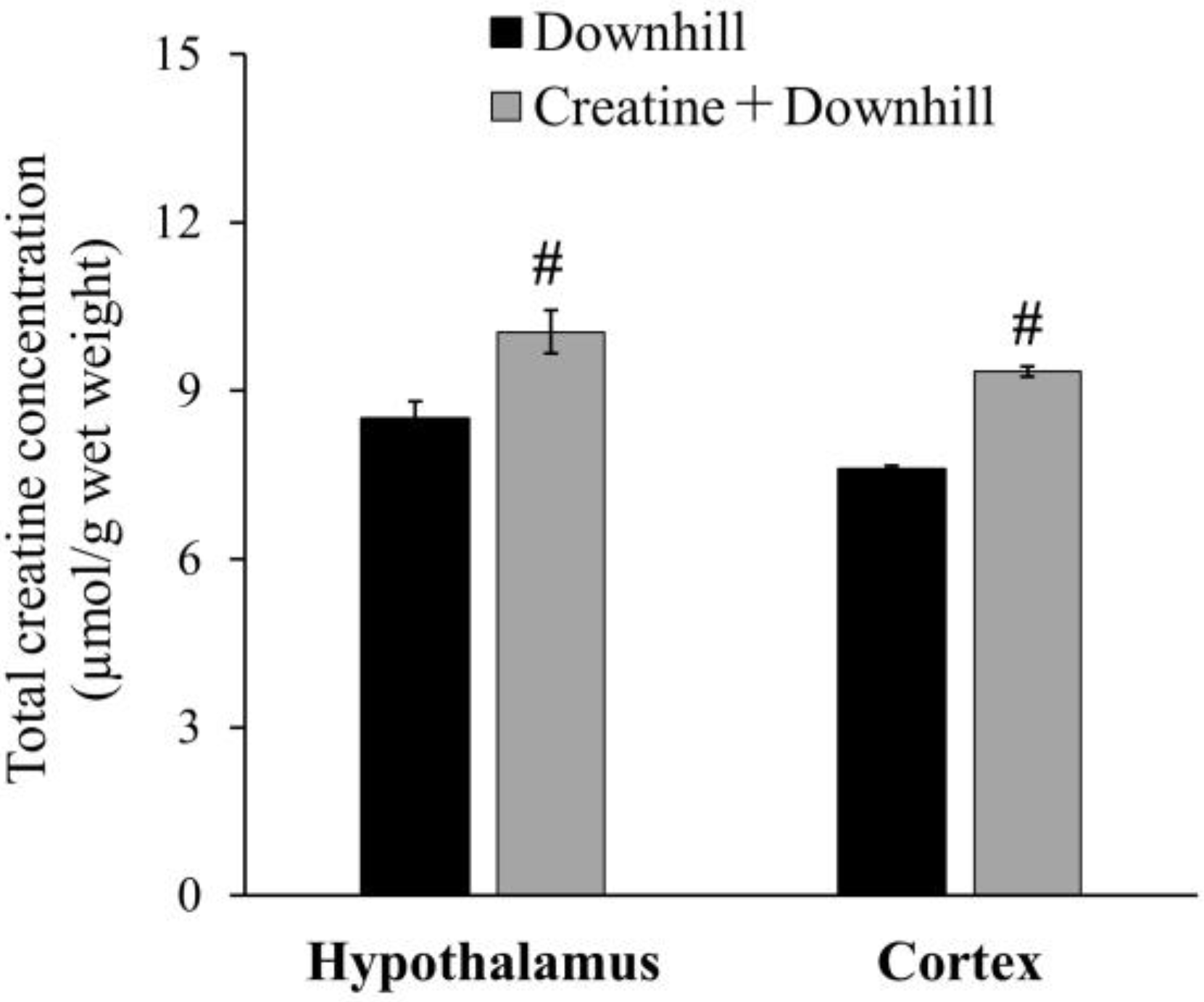
The total creatine concentration in the brain is shown in Figure 3. The Creatine + Downhill group had significantly higher values in the hypothalamus and cortex than the Downhill group (18.1% ± 4.5% and 22.9% ± 1.2%, respectively).
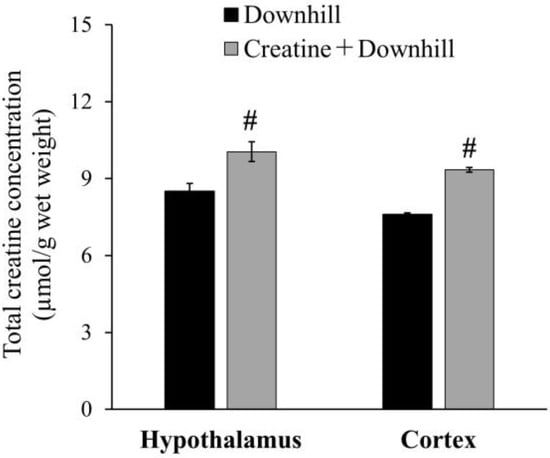
Figure 3.
Total creatine concentration in the brain of the Downhill group (black bar, n = 6) and the Creatine + Downhill group (gray bar, n = 6). Values are the means ± SEM. # indicates a significant difference from the Downhill group in the equivalent brain region (p < 0.05).
3.3. Experiment 1-3
Six mice in the Downhill group and eight mice in the Creatine + Downhill group were able to run downhill for 100 min or more (for 120 min at 22 m/min in all mice). After grouping, the food intake of each group was 2.3 ± 0.1 g/day in the Downhill group and 2.2 ± 0.0 g/day in the Creatine + Downhill group. The body weight just before the downhill running was 23.2 ± 0.2 g in the Downhill group and 22.8 ± 0.2 g in the Creatine + Downhill group. No significant differences in these indices were seen between the two groups.
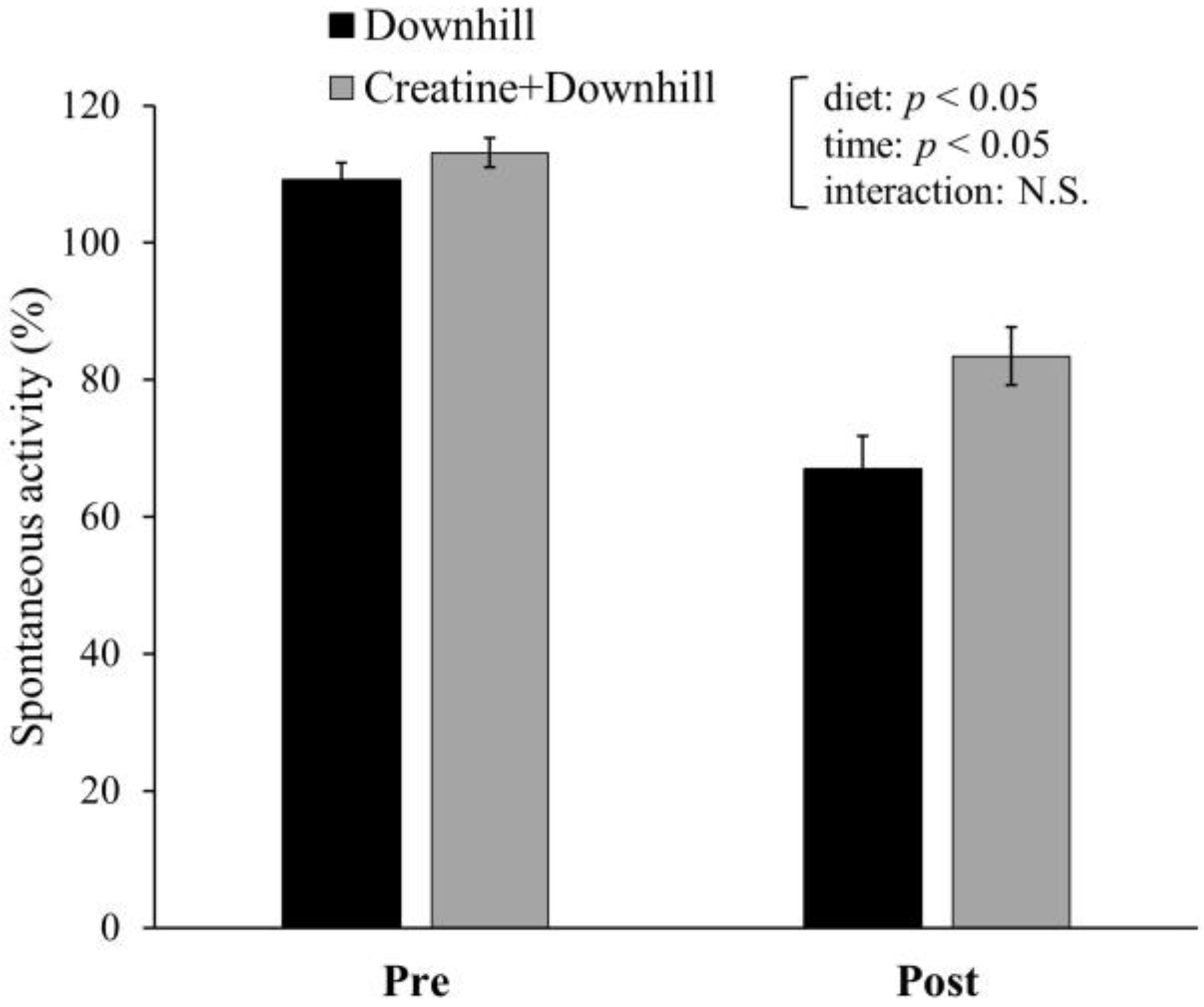
Spontaneous activity for 12 h prior to (Pre) and after (Post) the downhill running is shown in Figure 4. Significant main effects were found for time and diet. Spontaneous activity significantly decreased after downhill running. The Creatine + Downhill group maintained a significantly higher level of spontaneous activity than the Downhill group. There was no interaction between time and diet.
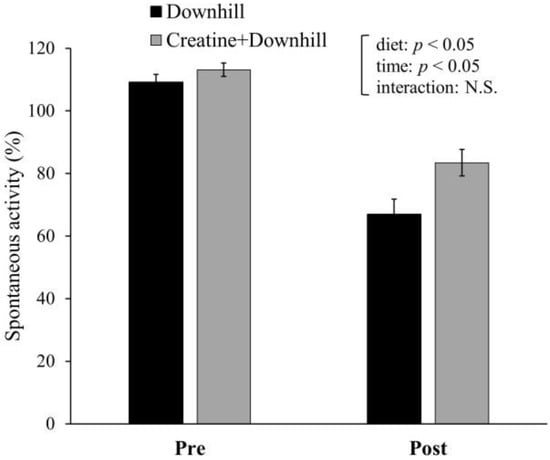
Figure 4.
Spontaneous activity before and after downhill running in the Downhill group (black bar, n = 6) and the Creatine + Downhill group (gray bar, n = 8). Values are the means ± SEM. The results of 2 × 2 repeated-measurement ANOVA (statistical analysis for main effects and interaction) are presented. N.S.: Not significant.
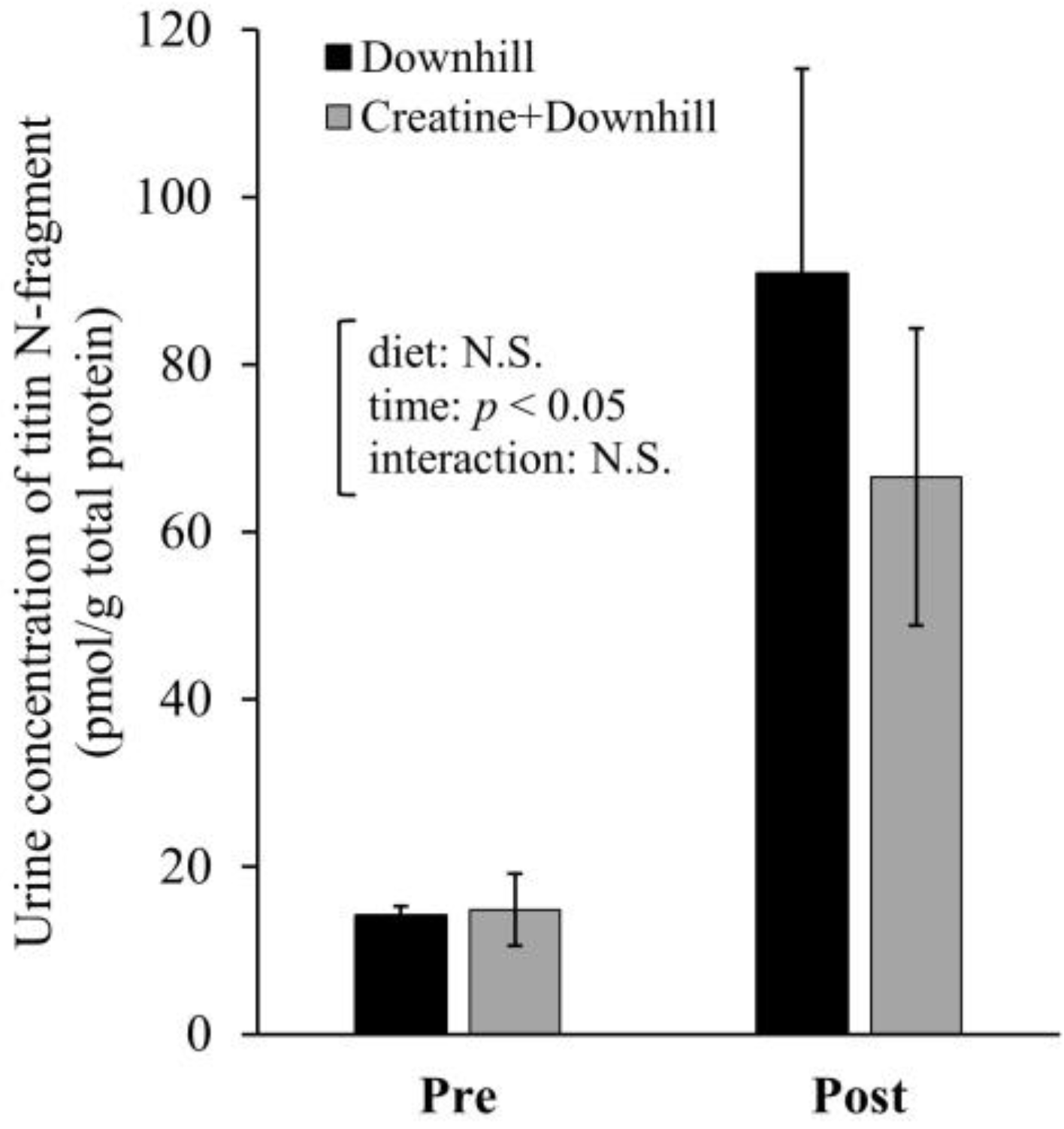
The urine concentration of titin N-fragment at approximately 96 h before (Pre) and 24 h after (Post) the downhill running is shown in Figure 5. The analysis showed a significant main effect for time. The urine concentration of titin N-fragment significantly increased after downhill running. No significant main effect for diet or interaction between time and diet were observed.
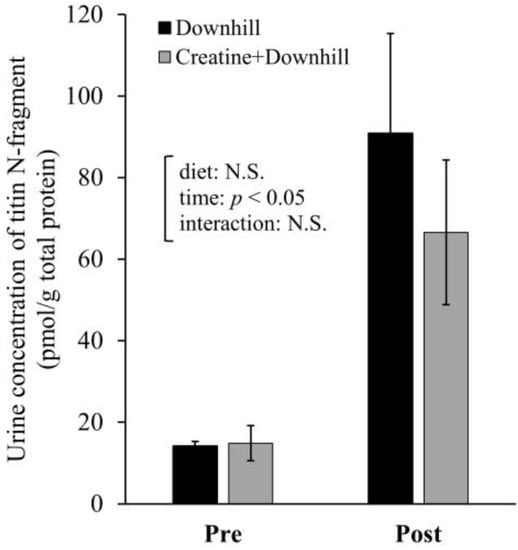
Figure 5.
Urine concentration of titin N-fragment before and after downhill running in the Downhill group (black bar, n = 5) and the Creatine + Downhill group (gray bar, n = 6). Values are the means ± SEM. The results of 2 × 2 repeated-measurement ANOVA (statistical analysis for main effects and interaction) are presented. N.S.: Not significant.
3.4. Experiment 2
Ten mice in the Downhill group and ten mice in the Creatine + Downhill group were able to run downhill for 100 min or more (for 120 min at 22 m/min in all mice). After grouping, the food intake of each group was 3.0 ± 0.1 g/day in the Rest group, 2.9 ± 0.1 g/day in the Downhill group, and 2.7 ± 0.0 g/day in the Creatine + Downhill group. The body weight just before the downhill running was 21.4 ± 0.3 g in the Rest group, 21.2 ± 0.2 g in the Downhill group, and 21.4 ± 0.1 g in the Creatine + Downhill group. No significant differences in these indices were seen between the three groups.
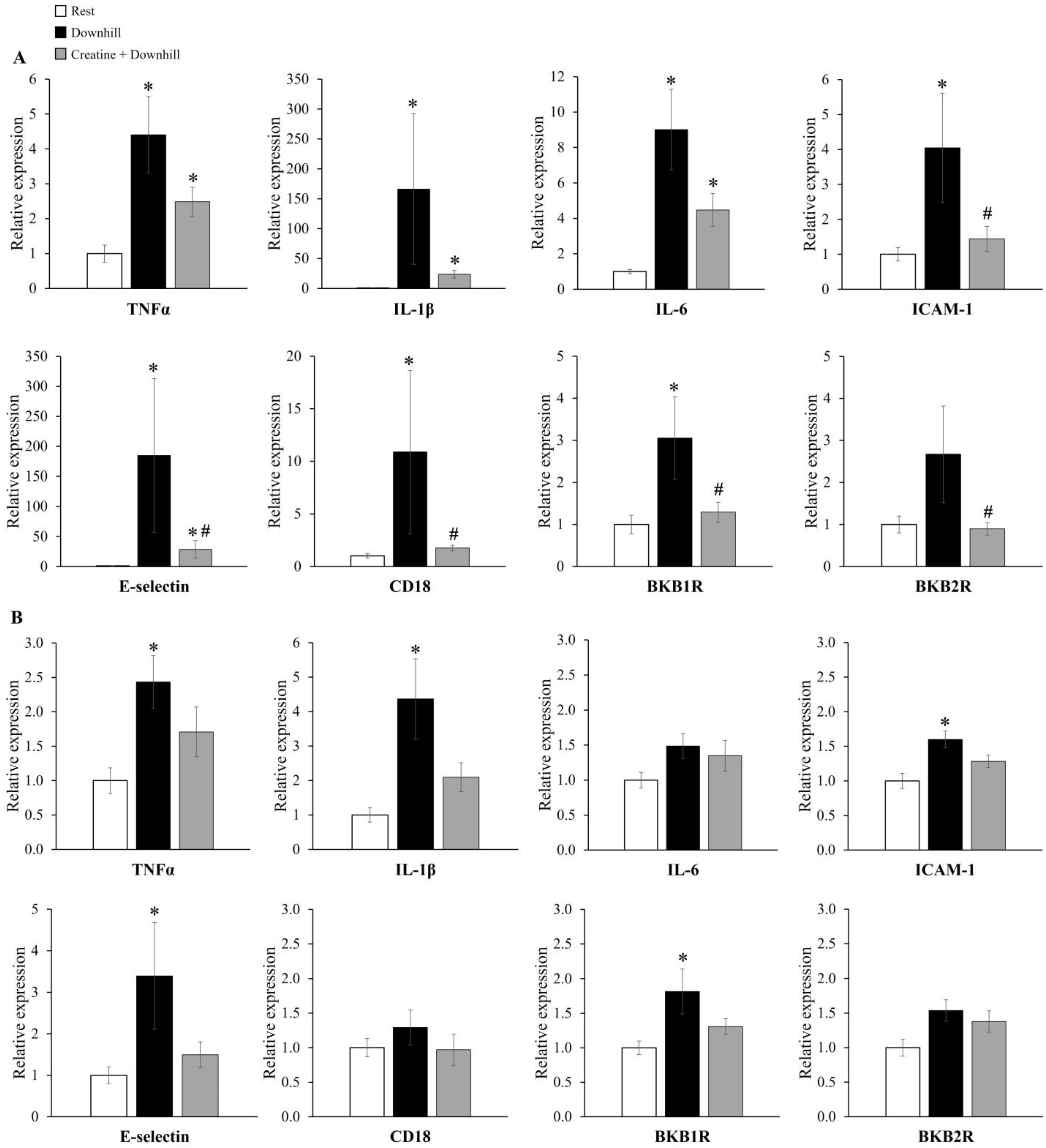
The mRNA expression levels of inflammatory genes in skeletal muscle are shown in Figure 6. In soleus muscle (Figure 6A), the mRNA expressions of TNFα, IL-1β, IL-6, ICAM-1, E-selectin, CD18, and BKB1R were significantly higher in the Downhill group than in the Rest group, while those of ICAM-1, E-selectin, CD18, and BKB1R were significantly lower in the Creatine + Downhill group than in the Downhill group. In plantaris muscle (Figure 6B), the mRNA expressions of TNFα, IL-1β, ICAM-1, E-selectin, and BKB1R were significantly higher in the Downhill group than in the Rest group. On the other hand, no significant differences in these markers were found between the Rest group and the Creatine + Downhill group. No significant differences in the mRNA expressions of IL-6, CD18, and BKB2R in plantaris muscle were observed among the three groups. The number of cases in the Downhill group is 10, except for the E-selectin in plantaris muscle (the mRNA expression level of the E-selectin in plantaris muscle of one mouse in the Downhill group could not be quantified).
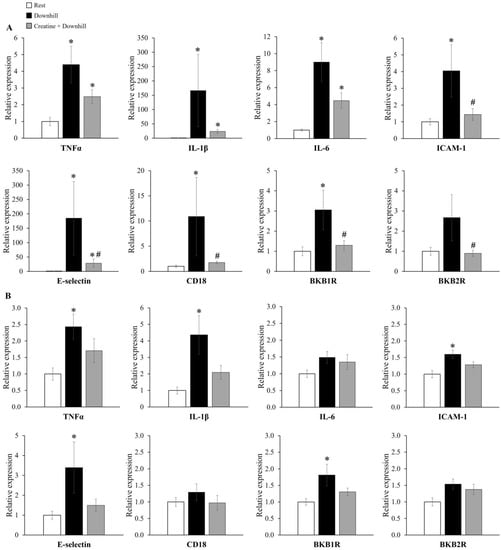
Figure 6.
Relative mRNA expressions of genes coding inflammatory molecules in the soleus (A) and plantaris (B) muscles of the Rest group (white bar, n = 12), the Downhill group (black bar, n = 9–10), and the Creatine + Downhill group (gray bar, n = 10). Values are the means ± SEM. * indicates a significant difference from the Rest group (p < 0.05). # indicates a significant difference from the Downhill group (p < 0.05).
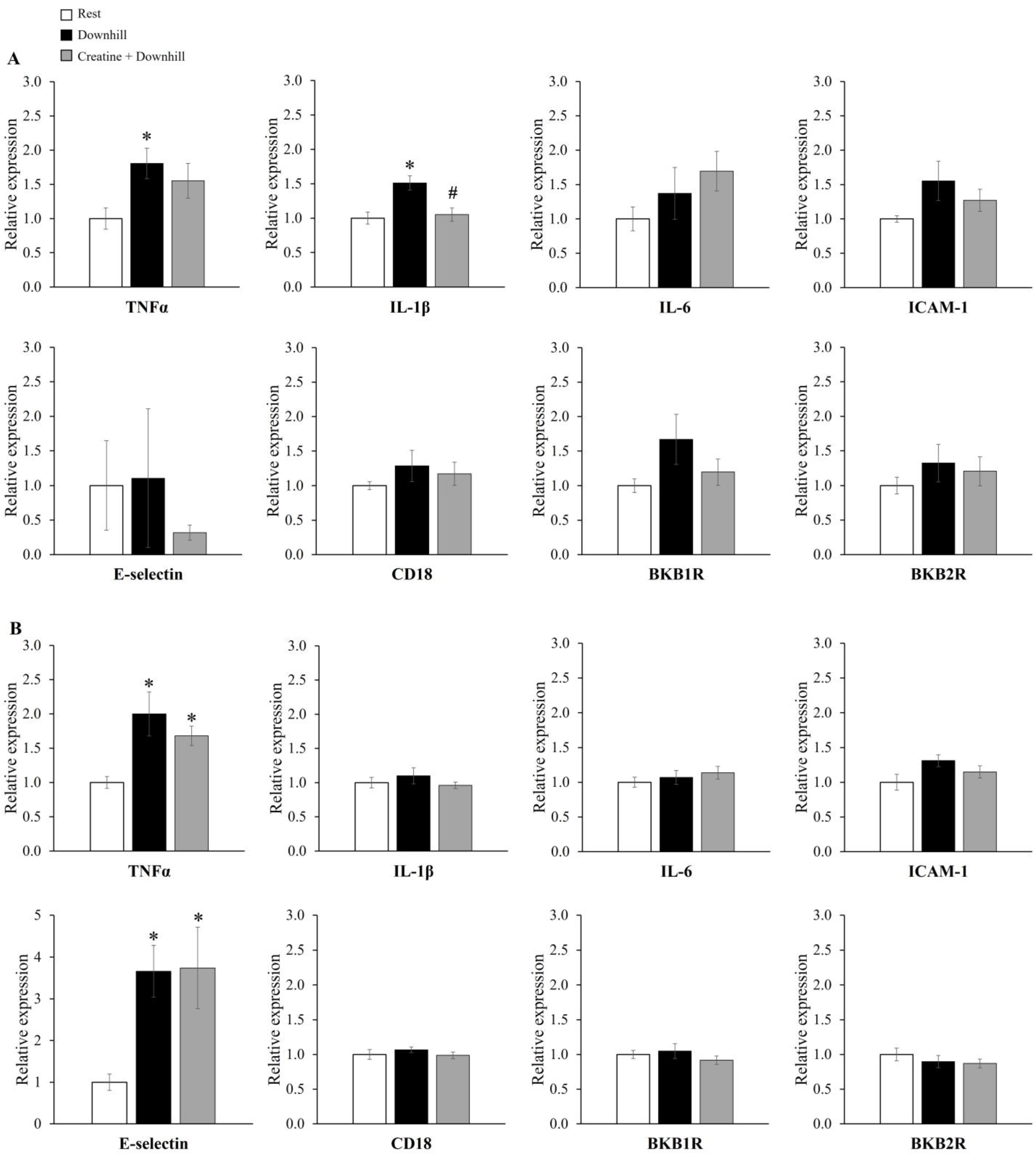
The mRNA expression levels of inflammatory genes in the brain are shown in Figure 7. The mRNA level of IL-1β in the hypothalamus was significantly higher in the Downhill group than in the Rest group, while it was significantly lower in the Creatine + Downhill group than in the Downhill group (Figure 7A). The mRNA level of TNFα in the hypothalamus was significantly higher in the Downhill group than in the Rest group, while no significant difference was seen between the Creatine + Downhill group and the Rest group. Regarding the mRNA levels of TNFα and E-selectin in the cortex (Figure 7B), the Downhill and Creatine + Downhill groups had significantly higher values than the Rest group, while no significant difference was observed between the Downhill group and the Creatine + Downhill group. No significant differences in the other markers were found among the three groups.
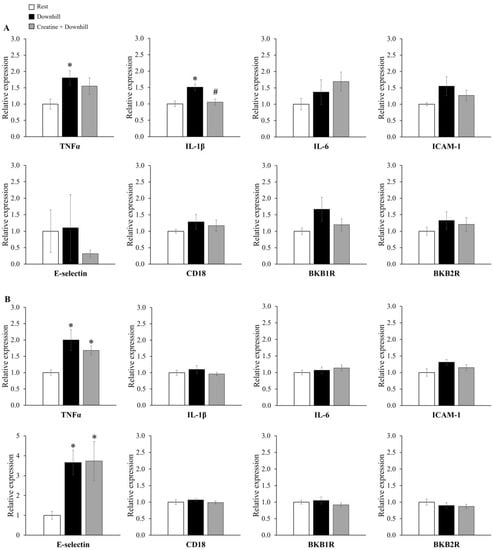
Figure 7.
Relative mRNA expressions of genes coding inflammatory molecules in the hypothalamus (A) and cortex (B) of the Rest group (white bar, n = 12), the Downhill group (black bar, n = 10), and the Creatine + Downhill group (gray bar, n = 10). Values are the means ± SEM. * indicates a significant difference from the Rest group (p < 0.05). # indicates a significant difference from the Downhill group (p < 0.05).
4. Discussion
To test the hypothesis that creatine supplementation alleviates fatigue after eccentric exercise through suppressive effects on myofibrillar damage and/or inflammation, we examined the effects of creatine supplementation on various indices using downhill running as an eccentric exercise model. The results showed that spontaneous activity significantly decreased after downhill running and creatine supplementation maintained a significantly higher level of spontaneous activity. In addition, creatine supplementation partially suppressed the downhill-running-induced increase in inflammatory gene expressions in both skeletal muscle and brain tissue. On the other hand, creatine supplementation did not clearly influence the myofibrillar damage index. These results suggest that creatine may be useful for alleviating fatigue after eccentric exercise and that its inhibitory effects on inflammatory responses in skeletal muscle and brain tissue may be involved in its mechanism of action.
The increased expression of adhesion molecules, such as ICAM-1 and E-selectin, is observed in tissues undergoing inflammation. These molecules induce the migration of leukocytes into tissues [41,42,43]. In addition, since CD18 is a receptor of adhesion molecules specifically expressed on the leukocyte surface regardless of the type of leukocyte, the mRNA expression level of the gene encoding CD18 has been used as an indicator of the extent of leukocyte infiltration into tissues [44,45]. In pulmonary vascular endothelial cells, creatine inhibited the increase in ICAM-1 and E-selectin expression [29]. In this study, creatine supplementation inhibited the downhill-running-induced increase in the mRNA expression of genes coding for ICAM-1, E-selectin, and CD18 in soleus muscle (Figure 6A), suggesting that creatine prevented leukocyte infiltration into the skeletal muscle by suppressing the increase in the expression of adhesion molecules. Pizza et al. reported that CD18-mediated leukocyte infiltration into skeletal muscle after eccentric exercise delayed the recovery of muscle strength [13]. Among various kinds of leukocytes, neutrophils in particular infiltrate skeletal muscle at the earliest stage after eccentric exercise and deteriorate muscle contractile function by secreting proteases and acids [46,47]. Therefore, the inhibitory effect on neutrophil infiltration into skeletal muscle is thought to be one of the mechanisms responsible for the anti-fatigue effect of creatine.
BKB1R and BKB2R are receptors for bradykinins and their metabolites [48]. They promote vascular permeability and edema formation during inflammation [49,50]. BKB1R is reported to be poorly expressed under normal conditions, but its expression is increased at the mRNA level during tissue injury [48,51]. BKB2R is thought to mediate delayed-onset muscle soreness [52,53]. In this study, creatine supplementation inhibited the downhill-running-induced increase in BKB1R mRNA expression in soleus muscle (Figure 6A). Thus, creatine supplementation may play a preventive role in skeletal muscle vascular permeability and edema formation via the suppression of BKB1R expression.
As shown in Figure 6, the downhill-running-induced changes in gene expression appeared to be more pronounced in soleus muscle than in plantaris muscle. Regarding all mRNA expressions, the extent of the downhill-running-induced increase was greater in soleus muscle than in plantaris muscle. For example, the extent of the increase in IL-1β was 166.3 ± 125.8 in soleus muscle and 4.4 ± 1.2 in plantaris muscle with the means ± SEM. Since the downhill running in this study was a low-intensity exercise that could be sustained for 120 min, the soleus muscle, which has a predominance of slow-twitch muscle fibers, may have been preferentially mobilized over the plantaris muscle, which has a predominance of fast-twitch muscle fibers; this would be in accordance with the size principle. In addition, the increase in the total creatine concentration seemed greater in the soleus muscle (15.4% ± 3.9%) than in the plantaris muscle (9.7% ± 3.5%), and the inhibitory effect of creatine supplementation on the downhill-running-induced changes in mRNA expression was statistically significant in the soleus muscle. Although creatine has been used as a supplement mainly by power/strength athletes [22], this study shows that creatine also alleviates fatigue after low-intensity eccentric exercise, which is loaded mainly on slow-twitch muscles. These results suggest that creatine may be useful for endurance athletes and non-athletic individuals who perform low-intensity exercise.
Inflammatory cytokines in brain tissue are considered to be a cause of feelings of tiredness after eccentric exercise [18]. Carmichael et al. reported that acute downhill running increased brain IL-1β expression and decreased spontaneous activity, and that the intracerebroventricular administration of an IL-1 receptor antagonist suppressed the decrease in spontaneous activity [19,20]. In the present study, creatine supplementation inhibited the downhill-running-induced increase in IL-1β mRNA expression in the brain (Figure 7A). Therefore, creatine supplementation may have maintained the higher level of spontaneous activity by suppressing the increase in brain IL-1β expression.
In the present study, creatine supplementation significantly increased the total creatine concentration in the brain (Figure 3). Based on this finding, the increased presence of creatine in the brain may have directly suppressed the increase in IL-1β expression. The downhill-running-induced increase in brain IL-1β disappeared by eliminating brain macrophages with the intracerebroventricular administration of clodronate [54]. This suggests that the increased brain IL-1β level after downhill running is derived from brain macrophages. After physical and psychological stress, brain-cell-derived damage-associated molecular patterns (DAMPs) are released into the extracellular space and recognition of DAMPs by receptors such as Toll-like receptors (TLRs) can promote the expression of proinflammatory cytokines in macrophages [55]. Furthermore, a study in which creatine was applied to macrophages in vitro reported that creatine suppressed the expression of TLRs in macrophages [56]. Therefore, the increased presence of creatine may have inhibited IL-1β production by brain macrophages through the DAMPs-TLRs pathway.
In the present study, an inhibitory effect of creatine on brain inflammatory cytokines was found only for IL-1β in the hypothalamus. Among various kinds of inflammatory cytokines, IL-1β has been suggested to be a key factor in fatigue in various pathological conditions [21]. In addition, the hypothalamus is a brain region that integrally regulates behaviors, and increased IL-1β expression in the hypothalamus has been associated with behavioral abnormalities in various animal models [57,58,59]. Based on these reports, we think that IL-1β in the hypothalamus has a significant effect on behavior, and the suppression of its expression can be considered as part of the mechanism of action of creatine.
A recent meta-analysis of human studies evaluating plasma creatine kinase activity and the lactate dehydrogenase level did not provide a clear conclusion as to the effect of creatine supplementation on muscle damage [27]. These markers are useful because creatine kinase and lactate dehydrogenase in skeletal muscle are released into the blood when the membranes of muscle cells are damaged [60]. Strictly speaking, therefore, they are markers of membrane damage in skeletal muscle and are not directly related to muscle function. On the other hand, the titin N-fragment is strongly related to the muscle contractile function because it reflects the degree of degradation of titin, which exerts tension during eccentric contraction [36,37,38,39]. Therefore, we used the urine concentration of titin N-fragment as an indicator of myofibrillar damage. In the present study, the effect of creatine supplementation on the urine concentration of titin N-fragment was not clear (Figure 5). We believe that no clear difference was observed because of large individual differences in the effects of downhill running on myofibrillar proteins.
Although creatine supplementation is known to enhance high-intensity exercise performance and muscle hypertrophy [22], we think that these actions are not involved in the anti-fatigue effect of creatine supplementation in this study. The reason is that the intensity of the downhill running and spontaneous activity in this study was presumed to be low. Therefore, the anti-inflammatory actions of creatine observed in this study are more likely to be involved. Furthermore, to the best of our knowledge, no intervention capable of mitigating eccentric-exercise-induced inflammation in the brain has been reported. We firstly discovered that creatine supplementation can mitigate brain inflammation. Fatigue may not be fully alleviated, since feelings of tiredness can remain even if muscle dysfunction is mitigated. In this study, creatine supplementation exerted beneficial effects in both skeletal muscle and the brain, making it a promising new approach to deal with post-exercise fatigue. Since creatine is safe and has some degree of recognition [22,24], this study would have a significant impact with a high potential for practical application.
We did not directly measure the feeling of tiredness and muscle function, which are essential components of fatigue, although we made reasonable assumptions about these parameters based on the data. Future research should examine the effect of creatine supplementation on these parameters.
5. Conclusions
In this study, spontaneous activity significantly decreased after eccentric exercise and creatine supplementation maintained a significantly higher level of spontaneous activity. In addition, inhibitory effects on inflammation in skeletal muscle and brain tissue may be involved in mechanisms of action of creatine. The anti-inflammatory effects of creatine were pronounced in slow-twitch skeletal muscle, suggesting that creatine may be useful for relieving fatigue in endurance athletes and non-athletic individuals who perform low-intensity exercise.
Author Contributions
Conceptualization, Y.Y. and A.M.; methodology, Y.Y., S.Y., K.K. and A.M.; investigation, Y.Y.; writing—original draft preparation, Y.Y.; data curation, Y.Y., D.Y. and K.K.; writing—review and editing, Y.Y., S.Y., D.Y., A.M. and A.T.; supervision, A.M. and A.T.; project administration, Y.Y., A.M. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study is funded by Taisho Pharmaceutical Co., Ltd. and receives no external funding.
Institutional Review Board Statement
All animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee at Taisho Pharmaceutical Co., Ltd. (approval number: AN13636-Z00, AN13862-Z01, AN13976-Z00, AN14033-Z00).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings in this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Y.Y., S.Y., D.Y., A.M. and A.T. are employees of Taisho Pharmaceutical Co., Ltd. K.K. is a former employee of Taisho Pharmaceutical Co., Ltd.
References
- Byrne, C.; Twist, C.; Eston, R. Neuromuscular Function after Exercise-Induced Muscle Damage: Theoretical and Applied Implications. Sport. Med. 2004, 34, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Hody, S.; Croisier, J.L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric Muscle Contractions: Risks and Benefits. Front. Physiol. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.C.; Fyfe, J.J. Post-Exercise Cold Water Immersion Effects on Physiological Adaptations to Resistance Training and the Underlying Mechanisms in Skeletal Muscle: A Narrative Review. Front. Sport. Act. Living 2021, 3, 660291. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.A.; Bishop, D.J. Effects of Dietary Supplements on Adaptations to Endurance Training. Sport. Med. 2020, 50, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Teixeira, V.H.; Soares, J. Dietary Strategies to Recover from Exercise-Induced Muscle Damage. Int. J. Food Sci. Nutr. 2014, 65, 151–163. [Google Scholar] [CrossRef]
- Yamada, R.; Himori, K.; Tatebayashi, D.; Ashida, Y.; Ikezaki, K.; Miyata, H.; Kanzaki, K.; Wada, M.; Westerblad, H.; Yamada, T. Preconditioning Contractions Prevent the Delayed Onset of Myofibrillar Dysfunction after Damaging Eccentric Contractions. J. Physiol. 2018, 596, 4427–4442. [Google Scholar] [CrossRef]
- Tee, J.C.; Bosch, A.N.; Lambert, M.I. Metabolic Consequences of Exercise-Induced Muscle Damage. Sport. Med. 2007, 37, 827–836. [Google Scholar] [CrossRef]
- Kirwan, J.P.; del Aguila, L.F. Insulin Signalling, Exercise and Cellular Integrity. Biochem. Soc. Trans. 2003, 31, 1281–1285. [Google Scholar] [CrossRef]
- del Aguila, L.F.; Krishnan, R.K.; Ulbrecht, J.S.; Farrell, P.A.; Correll, P.H.; Lang, C.H.; Zierath, J.R.; Kirwan, J.P. Muscle Damage Impairs Insulin Stimulation of IRS-1, PI 3-Kinase, and Akt-Kinase in Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, 206–212. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Hickner, R.C.; Yarasheski, K.E.; Kohrt, W.M.; Wiethop, B.V.; Holloszy, J.O. Eccentric Exercise Induces Transient Insulin Resistance in Healthy Individuals. J. Appl. Physiol. 1992, 72, 2197–2202. [Google Scholar] [CrossRef]
- Hickner, R.C.; Mehta, P.M.; Dyck, D.; Devita, P.; Houmard, J.A.; Koves, T.; Byrd, P. Relationship between Fat-to-Fat-Free Mass Ratio and Decrements in Leg Strength after Downhill Running. J. Appl. Physiol. 2001, 90, 1334–1341. [Google Scholar] [CrossRef]
- Zehnder, M.; Muelli, M.; Buchli, R.; Kuehne, G.; Boutellier, U. Further Glycogen Decrease during Early Recovery after Eccentric Exercise despite a High Carbohydrate Intake. Eur. J. Nutr. 2004, 43, 148–159. [Google Scholar] [CrossRef]
- Pizza, F.X.; Peterson, J.M.; Baas, J.H.; Koh, T.J. Neutrophils Contribute to Muscle Injury and Impair Its Resolution after Lengthening Contractions in Mice. J. Physiol. 2005, 562, 899–913. [Google Scholar] [CrossRef]
- Chen, T.C.; Nosaka, K.; Tu, J.H. Changes in Running Economy Following Downhill Running. J. Sport. Sci. 2007, 25, 55–63. [Google Scholar] [CrossRef]
- Chen, T.C.; Nosaka, K.; Lin, M.J.; Chen, H.L.; Wu, C.J. Changes in Running Economy at Different Intensities Following Downhill Running. J. Sport. Sci. 2009, 27, 1137–1144. [Google Scholar] [CrossRef]
- Burt, D.G.; Lamb, K.; Nicholas, C.; Twist, C. Effects of Exercise-Induced Muscle Damage on Resting Metabolic Rate, Sub-Maximal Running and Post-Exercise Oxygen Consumption. Eur. J. Sport. Sci. 2014, 14, 337–344. [Google Scholar] [CrossRef]
- Burt, D.; Lamb, K.; Nicholas, C.; Twist, C. Lower-Volume Muscle-Damaging Exercise Protects against High-Volume Muscle-Damaging Exercise and the Detrimental Effects on Endurance Performance. Eur. J. Appl. Physiol. 2015, 115, 1523–1532. [Google Scholar] [CrossRef]
- Proschinger, S.; Freese, J. Neuroimmunological and Neuroenergetic Aspects in Exercise-Induced Fatigue. Exerc. Immunol. Rev. 2019, 25, 8–19. [Google Scholar]
- Carmichael, M.D.; Davis, J.M.; Murphy, E.A.; Brown, A.S.; Carson, J.A.; Mayer, E.; Ghaffar, A. Recovery of Running Performance Following Muscle-Damaging Exercise: Relationship to Brain IL-1β. Brain Behav. Immun. 2005, 19, 445–452. [Google Scholar] [CrossRef]
- Carmichael, M.D.; Davis, J.M.; Murphy, E.A.; Brown, A.S.; Carson, J.A.; Mayer, E.P.; Ghaffar, A. Role of Brain IL-1β on Fatigue after Exercise-Induced Muscle Damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, 1344–1348. [Google Scholar] [CrossRef]
- Roerink, M.E.; van der Schaaf, M.E.; Dinarello, C.A.; Knoop, H.; van der Meer, J.W.M. Interleukin-1 as a Mediator of Fatigue in Disease: A Narrative Review. J. Neuroinflam. 2017, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition Position Stand: Safety and Efficacy of Creatine Supplementation in Exercise, Sport, and Medicine. J. Int. Soc. Sport. Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The Creatine Kinase System and Pleiotropic Effects of Creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism during Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Shao, A.; Inoue, T.; Kreider, R.B. Analysis of the Efficacy, Safety, and Regulatory Status of Novel Forms of Creatine. Amino Acids 2011, 40, 1369–1383. [Google Scholar] [CrossRef]
- Guzun, R.; Timohhina, N.; Tepp, K.; Gonzalez-Granillo, M.; Shevchuk, I.; Chekulayev, V.; Kuznetsov, A.V.; Kaambre, T.; Saks, V.A. Systems Bioenergetics of Creatine Kinase Networks: Physiological Roles of Creatine and Phosphocreatine in Regulation of Cardiac Cell Function. Amino Acids 2011, 40, 1333–1348. [Google Scholar] [CrossRef]
- Northeast, B.; Clifford, T. The Effect of Creatine Supplementation on Markers of Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis of Human Intervention Trials. Int. J. Sport. Nutr. Exerc. Metab. 2021, 31, 276–291. [Google Scholar] [CrossRef]
- Cordingley, D.M.; Cornish, S.M.; Candow, D.G. Anti-Inflammatory and Anti-Catabolic Effects of Creatine Supplementation: A Brief Review. Nutrients 2022, 14, 544. [Google Scholar] [CrossRef]
- Nomura, A.; Zhang, M.; Sakamoto, T.; Ishii, Y.; Morishima, Y.; Mochizuki, M.; Kimura, T.; Uchida, Y.; Sekizawa, K. Anti-Inflammatory Activity of Creatine Supplementation in Endothelial Cells in Vitro. Br. J. Pharmacol. 2003, 139, 715–720. [Google Scholar] [CrossRef]
- Khanna, N.K.; Madan, B.R. Studies on the Anti-Inflammatory Activity of Creatine. Arch. Int. Pharmacodyn. Ther. 1978, 231, 340–350. [Google Scholar]
- Ohtsuki, S.; Tachikawa, M.; Takanaga, H.; Shimizu, H.; Watanabe, M.; Hosoya, K.; Terasaki, T. The Blood-Brain Barrier Creatine Transporter Is a Major Pathway for Supplying Creatine to the Brain. J. Cereb. Blood Flow. Metab. 2002, 22, 1327–1335. [Google Scholar] [CrossRef]
- Béard, E.; Braissant, O. Synthesis and Transport of Creatine in the CNS: Importance for Cerebral Functions. J. Neurochem. 2010, 115, 297–313. [Google Scholar] [CrossRef]
- Pazini, F.L.; Cunha, M.P.; Rodrigues, A.L.S. The Possible Beneficial Effects of Creatine for the Management of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 193–206. [Google Scholar] [CrossRef]
- Ainsley Dean, P.J.; Arikan, G.; Opitz, B.; Sterr, A. Potential for Use of Creatine Supplementation Following Mild Traumatic Brain Injury. Concussion 2017, 2, CNC34. [Google Scholar] [CrossRef]
- Schwane, J.A.; Armstrong, R.B. Effect of Training on Skeletal Muscle Injury from Downhill Running in Rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 969–975. [Google Scholar] [CrossRef]
- Hessel, A.L.; Lindstedt, S.L.; Nishikawa, K.C. Physiological Mechanisms of Eccentric Contraction and Its Applications: A Role for the Giant Titin Protein. Front. Physiol. 2017, 8, 70. [Google Scholar] [CrossRef]
- Rouillon, J.; Zocevic, A.; Leger, T.; Garcia, C.; Camadro, J.M.; Udd, B.; Wong, B.; Servais, L.; Voit, T.; Svinartchouk, F. Proteomics Profiling of Urine Reveals Specific Titin Fragments as Biomarkers of Duchenne Muscular Dystrophy. Neuromuscul. Disord. 2014, 24, 563–573. [Google Scholar] [CrossRef]
- Kanda, K.; Sakuma, J.; Akimoto, T.; Kawakami, Y.; Suzuki, K. Detection of Titin Fragments in Urine in Response to Exercise-Induced Muscle Damage. PLoS ONE 2017, 12, e0181623. [Google Scholar] [CrossRef]
- Inami, T.; Yamaguchi, S.; Ishida, H.; Kohtake, N.; Morito, A.; Yamada, S.; Shimomasuda, M.; Haramoto, M.; Nagata, N.; Murayama, M. Changes in Muscle Shear Modulus and Urinary Titin N-Terminal Fragment after Eccentric Exercise. J. Sport. Sci. Med. 2022, 21, 536–544. [Google Scholar] [CrossRef]
- Hultman, E.; Söderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle Creatine Loading in Men. J. Appl. Physiol. 1996, 81, 232–237. [Google Scholar] [CrossRef]
- Ou, Z.; Dolmatova, E.; Lassegue, B.; Griendling, K.K. B1- And B2-Integrins: Central Players in Regulating Vascular Permeability and Leukocyte Recruitment during Acute Inflammation. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, M.; Stege, H.; Grabbe, S.; Bros, M. B2 Integrins—Multi-Functional Leukocyte Receptors in Health and Disease. Int. J. Mol. Sci. 2020, 21, 1402. [Google Scholar] [CrossRef] [PubMed]
- Chase, S.D.; Magnani, J.L.; Simon, S.I. E-Selectin Ligands as Mechanosensitive Receptors on Neutrophils in Health and Disease. Ann. Biomed. Eng. 2012, 40, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Blythe, E.N.; Weaver, L.C.; Brown, A.; Dekaban, G.A. B2 Integrin CD11d/CD18: From Expression to an Emerging Role in Staged Leukocyte Migration. Front. Immunol. 2021, 12, 775447. [Google Scholar] [CrossRef]
- Hamada, K.; Vannier, E.; Sacheck, J.M.; Witsell, A.L.; Roubenoff, R. Senescence of Human Skeletal Muscle Impairs the Local Inflammatory Cytokine Response to Acute Eccentric Exercise. FASEB J. 2005, 19, 1–19. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, X.O.; della Gatta, P.A.; Nosaka, K. Muscle Damage and Inflammation during Recovery from Exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Pizza, F.X.; Mcloughlin, T.J.; Mcgregor, S.J.; Calomeni, E.P.; Gunning, W.T. Neutrophils Injure Cultured Skeletal Myotubes. Am. J. Physiol. Cell. Physiol. 2001, 281, 335–341. [Google Scholar] [CrossRef]
- Marceau, F.; Bachelard, H.; Bouthillier, J.; Fortin, J.P.; Morissette, G.; Bawolak, M.T.; Charest-Morin, X.; Gera, L. Bradykinin Receptors: Agonists, Antagonists, Expression, Signaling, and Adaptation to Sustained Stimulation. Int. Immunopharmacol. 2020, 82, 106305. [Google Scholar] [CrossRef]
- Ni, A.; Yin, H.; Agata, J.; Yang, Z.; Chao, L.; Chao, J. Overexpression of Kinin B1 Receptors Induces Hypertensive Response to Des-Arg9-Bradykinin and Susceptibility to Inflammation. J. Biol. Chem. 2003, 278, 219–225. [Google Scholar] [CrossRef]
- Todorov, A.G.; Andrade, D.; Pesquero, J.B.; Araujo, R.d.C.; Bader, M.; Stewart, J.; Gera, L.; Müller-Esterl, W.; Morandi, V.; Goldenberg, R.C.S.; et al. Trypanosoma Cruzi Induces Edematogenic Responses in Mice and Invades Cardiomyocytes and Endothelial Cells in Vitro by Activating Distinct Kinin Receptor (B1/B2) Subtypes. FASEB J. 2003, 17, 73–75. [Google Scholar] [CrossRef]
- Bortone, F.; Santos, H.A.; Albertini, R.; Pesquero, J.B.; Costa, M.S.; Silva, J.A. Low Level Laser Therapy Modulates Kinin Receptors mRNA Expression in the Subplantar Muscle of Rat Paw Subjected to Carrageenan-Induced Inflammation. Int. Immunopharmacol. 2008, 8, 206–210. [Google Scholar] [CrossRef]
- Mizumura, K.; Taguchi, T. Delayed Onset Muscle Soreness: Involvement of Neurotrophic Factors. J. Physiol. Sci. 2016, 66, 43–52. [Google Scholar] [CrossRef]
- Nagahisa, H.; Ikezaki, K.; Yamada, R.; Yamada, T.; Miyata, H. Preconditioning Contractions Suppress Muscle Pain Markers after Damaging Eccentric Contractions. Pain. Res. Manag. 2018, 2018, 3080715. [Google Scholar] [CrossRef]
- Carmichael, M.D.; Davis, J.M.; Murphy, E.A.; Carson, J.A.; van Rooijen, N.; Mayer, E.; Ghaffar, A. Role of Brain Macrophages on IL-1β and Fatigue Following Eccentric Exercise-Induced Muscle Damage. Brain Behav. Immun. 2010, 24, 564–568. [Google Scholar] [CrossRef]
- Serna-Rodríguez, M.F.; Bernal-Vega, S.; de la Barquera, J.A.O.S.; Camacho-Morales, A.; Pérez-Maya, A. The Role of Damage Associated Molecular Pattern Molecules (DAMPs) and Permeability of the Blood-Brain Barrier in Depression and Neuroinflammation. J. Neuroimmunol. 2022, 371, 577951. [Google Scholar] [CrossRef]
- Leland, K.M.; McDonald, T.L.; Drescher, K.M. Effect of Creatine, Creatinine, and Creatine Ethyl Ester on TLR Expression in Macrophages. Int. Immunopharmacol. 2011, 11, 1341–1347. [Google Scholar] [CrossRef]
- Damm, J.; Wiegand, F.; Harden, L.M.; Gerstberger, R.; Rummel, C.; Roth, J. Fever, Sickness Behavior, and Expression of Inflammatory Genes in the Hypothalamus after Systemic and Localized Subcutaneous Stimulation of Rats with the Toll-like Receptor 7 Agonist Imiquimod. Neuroscience 2012, 201, 166–183. [Google Scholar] [CrossRef]
- Harden, L.M.; du Plessis, I.; Roth, J.; Loram, L.C.; Poole, S.; Laburn, H.P. Differences in the Relative Involvement of Peripherally Released Interleukin (IL)-6, Brain IL-1β and Prostanoids in Mediating Lipopolysaccharide-Induced Fever and Sickness Behavior. Psychoneuroendocrinology 2011, 36, 608–622. [Google Scholar] [CrossRef]
- Pereira, B.C.; da Rocha, A.L.; Pauli, J.R.; Ropelle, E.R.; de Souza, C.T.; Cintra, D.E.; Sant’Ana, M.R.; da Silva, A.S.R. Excessive Eccentric Exercise Leads to Transitory Hypothalamic Inflammation, which May Contribute to the Low Body Weight Gain and Food Intake in Overtrained Mice. Neuroscience 2015, 311, 231–242. [Google Scholar] [CrossRef]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical Markers of Muscular Damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).