Antiproliferative Effect of Plant-Derived Bioactive Compounds Endowed with Antioxidant Activity on Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
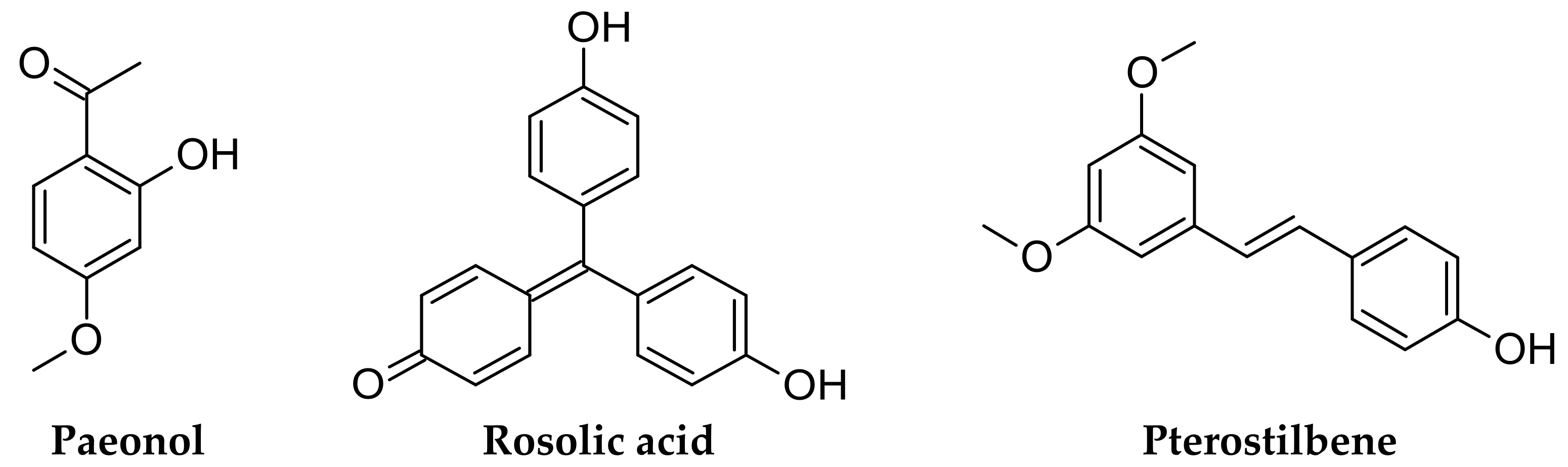
2.1. Chemicals
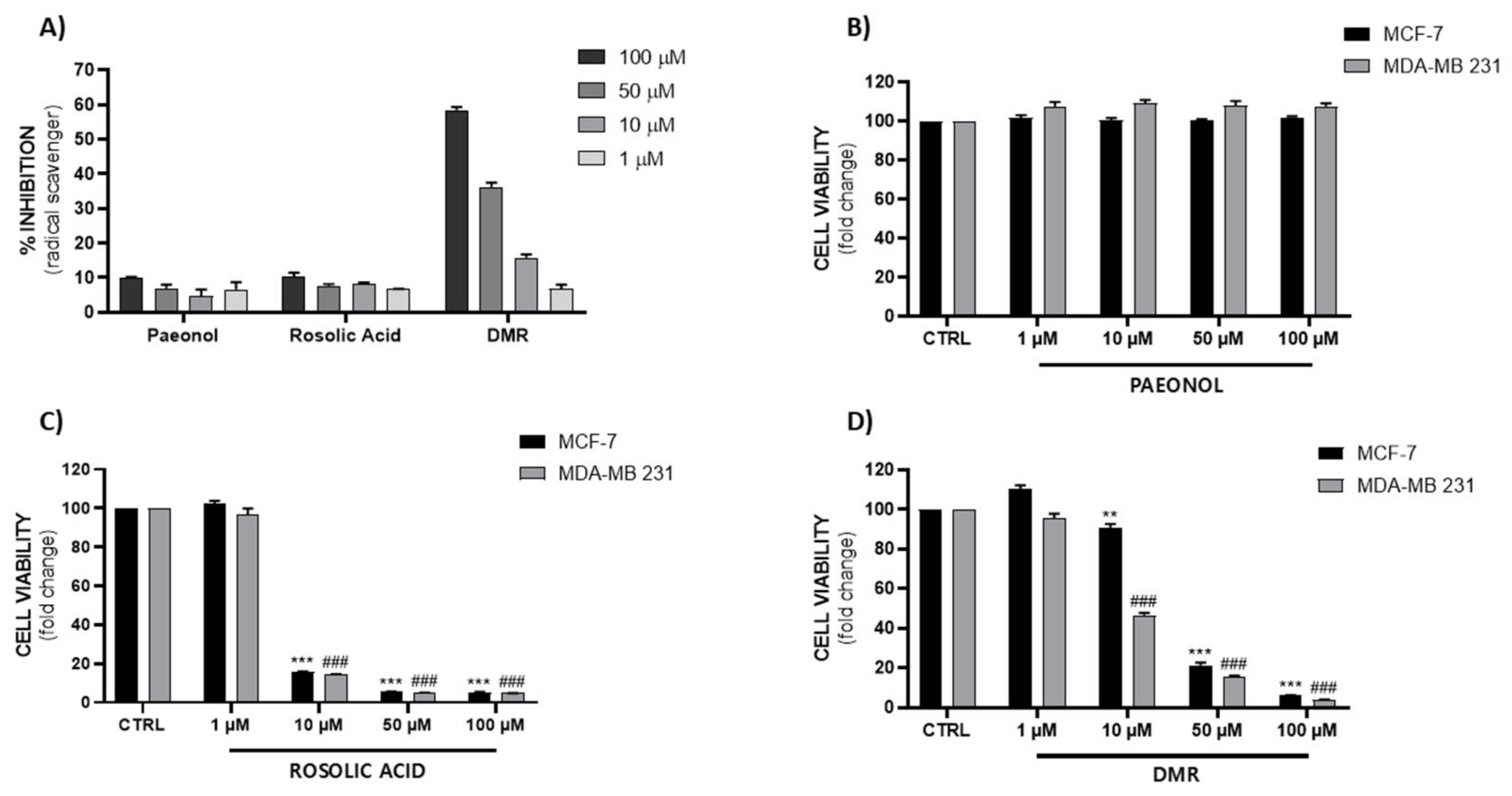
2.2. Evaluation of Free Radical Scavenger Activity (DPPH)
2.3. Cell Culture and Treatments
2.4. Cell Viability
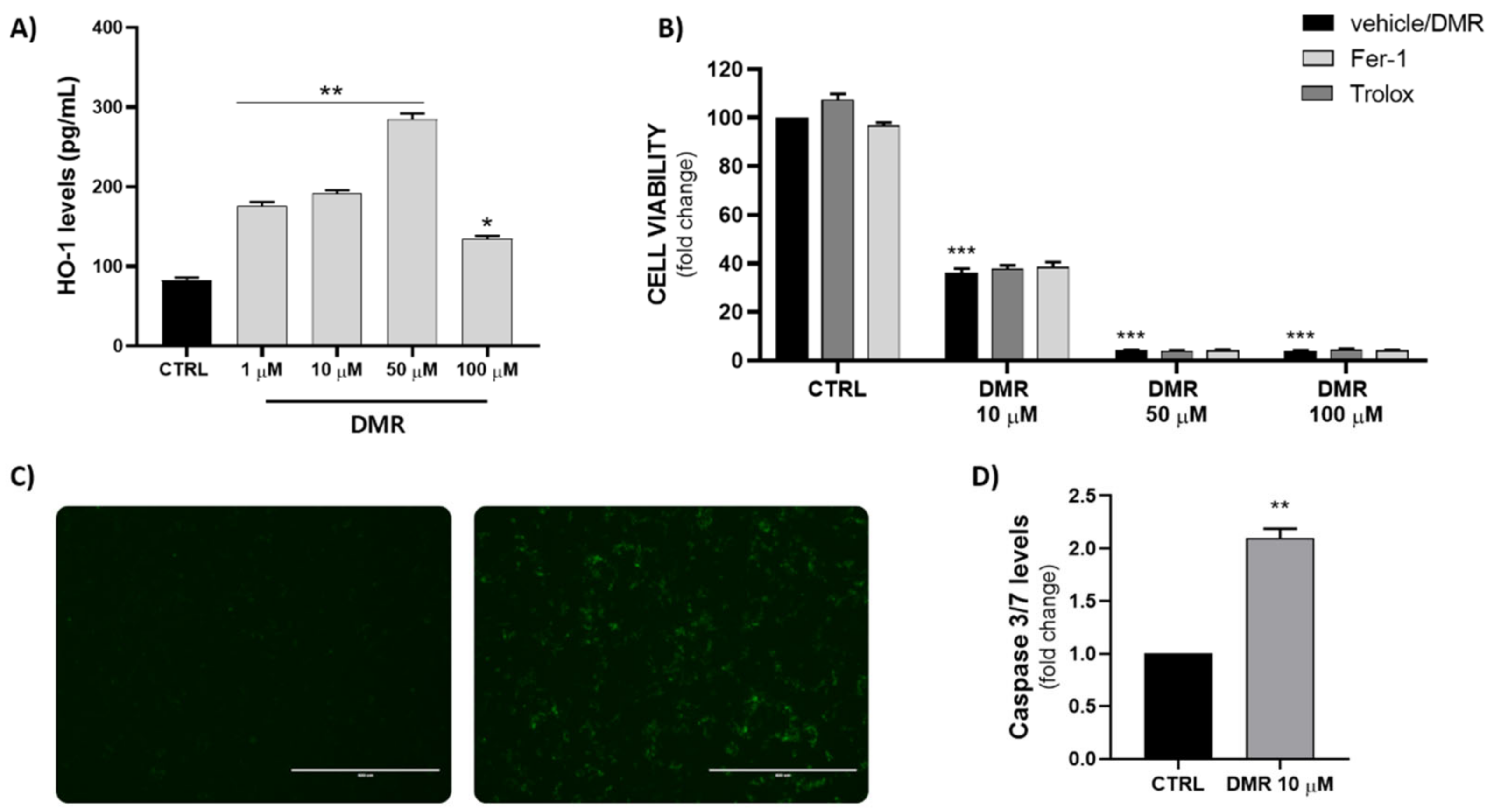
2.5. Measurement of HO-1 Levels (ELISA)
2.6. Measurement of Activated Caspase 3/7
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fallica, A.N.; Sorrenti, V.; D’Amico, A.G.; Salerno, L.; Romeo, G.; Intagliata, S.; Consoli, V.; Floresta, G.; Rescifina, A.; D’Agata, V.; et al. Discovery of Novel Acetamide-Based Heme Oxygenase-1 Inhibitors with Potent. J. Med. Chem. 2021, 64, 13373–13393. [Google Scholar] [CrossRef] [PubMed]
- Salerno, L.; Vanella, L.; Sorrenti, V.; Consoli, V.; Ciaffaglione, V.; Fallica, A.N.; Canale, V.; Zajdel, P.; Pignatello, R.; Intagliata, S. Novel mutual prodrug of 5-fluorouracil and heme oxygenase-1 inhibitor (5-FU/HO-1 hybrid): Design and preliminary. J. Enzym. Inhib. Med. Chem. 2021, 36, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.N.; Vukomanovic, D.; Vlahakis, J.Z.; Szarek, W.A.; Nakatsu, K.; Jia, Z. Structural insights into human heme oxygenase-1 inhibition by potent and selective azole-based compounds. J. R. Soc. Interface 2013, 10, 20120697. [Google Scholar] [CrossRef] [PubMed]
- Tertil, M.; Golda, S.; Skrzypek, K.; Florczyk, U.; Weglarczyk, K.; Kotlinowski, J.; Maleszewska, M.; Czauderna, S.; Pichon, C.; Kieda, C.; et al. Nrf2-heme oxygenase-1 axis in mucoepidermoid carcinoma of the lung: Antitumoral effects associated with down-regulation of matrix metalloproteinases. Free Radic. Biol. Med. 2015, 89, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; D’Amico, A.G.; Barbagallo, I.; Consoli, V.; Grosso, S.; Vanella, L. Tin Mesoporphyrin Selectively Reduces Non-Small-Cell Lung Cancer Cell Line A549 Proliferation by Interfering with Heme Oxygenase and Glutathione Systems. Biomolecules 2021, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, L.; Sun, C.; Zhang, B.; Li, J.; Sun, J.; Zhang, Y.; Sun, D. Paeonol alleviates epirubicin-induced renal injury in mice by regulating Nrf2 and NF-κB pathways. Eur. J. Pharm. 2017, 795, 84–93. [Google Scholar] [CrossRef]
- Amin, K.N.; Palanisamy, R.; Sarada, D.V.L.; Ali, D.; Suzuki, T.; Ramkumar, K.M. Effect of Rosolic acid on endothelial dysfunction under ER stress in pancreatic microenvironment. Free Radic. Res. 2021, 55, 698–713. [Google Scholar] [CrossRef]
- Zeng, Q.; Lian, W.; Wang, G.; Qiu, M.; Lin, L.; Zeng, R. Pterostilbene induces Nrf2/HO-1 and potentially regulates NF-κB and JNK-Akt/mTOR signaling in ischemic brain injury in neonatal rats. 3 Biotech. 2020, 10, 192. [Google Scholar] [CrossRef]
- Lau, C.H.; Chan, C.M.; Chan, Y.W.; Lau, K.M.; Lau, T.W.; Lam, F.C.; Law, W.T.; Che, C.T.; Leung, P.C.; Fung, K.P.; et al. Pharmacological investigations of the anti-diabetic effect of Cortex Moutan and its active component paeonol. Phytomedicine 2007, 14, 778–784. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Du, L.-D.; Lu, Y. Natural Small Molecule Drugs from Plants; Paeonol; Springer: Berlin/Heidelberg, Germany, 2018; pp. 439–444. [Google Scholar] [CrossRef]
- Sun, Z.; Du, J.; Hwang, E.; Yi, T.H. Paeonol extracted from Paeonia suffruticosa Andr. ameliorated UVB-induced skin photoaging via DLD/Nrf2/ARE and MAPK/AP-1 pathway. Phytother. Res. 2018, 32, 1741–1749. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.X.; Li, L.L.; Cao, Y.Z.; Geng, Y.D.; Feng, X.J.; Wang, A.Y.; Chen, Z.L.; Lu, Y.; Shen, A.Z. Paeonol Suppresses Proliferation and Motility of Non-Small-Cell Lung Cancer Cells by Disrupting STAT3/NF-κB Signaling. Front. Pharm. 2020, 11, 572616. [Google Scholar] [CrossRef] [PubMed]
- Naresh Amin, K.; Rajagru, P.; Sarkar, K.; Ganesh, M.R.; Suzuki, T.; Ali, D.; Kunka Mohanram, R. Pharmacological Activation of Nrf2 by Rosolic Acid Attenuates Endoplasmic Reticulum Stress in Endothelial Cells. Oxid. Med. Cell. Longev. 2021, 2021, 2732435. [Google Scholar] [CrossRef]
- Foresti, R.; Hoque, M.; Monti, D.; Green, C.J.; Motterlini, R. Differential activation of heme oxygenase-1 by chalcones and rosolic acid in endothelial cells. J. Pharm. Exp. Ther. 2005, 312, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Angela, L.D.; Qiao, S.; Lesson, J.L.; de la Vega, M.R.; Park, S.L.; Seanez, C.M.; Gokhale, V.; Cabello, C.M.; Wondrak, G.T. The quinone methide aurin is a heat shock response inducer that causes proteotoxic stress and Noxa-dependent apoptosis in malignant melanoma cells. J. Biol. Chem. 2015, 290, 1623–1638. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Medina, I.; Ortega, A.; Carretero, J.; Baño, M.C.; Obrador, E.; Estrela, J.M. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 2002, 33, 387–398. [Google Scholar] [CrossRef]
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228. [Google Scholar] [CrossRef]
- Nutakul, W.; Sobers, H.S.; Qiu, P.; Dong, P.; Decker, E.A.; McClements, D.J.; Xiao, H. Inhibitory effects of resveratrol and pterostilbene on human colon cancer cells: A side-by-side comparison. J. Agric. Food Chem. 2011, 59, 10964–10970. [Google Scholar] [CrossRef]
- Tan, K.T.; Chen, P.W.; Li, S.; Ke, T.M.; Lin, S.H.; Yang, C.C. Pterostilbene inhibits lung squamous cell carcinoma growth. Oncol. Lett. 2019, 18, 1631–1640. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Chen, B.; Ning, M.; Yang, G. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 2012, 17, 4672–4683. [Google Scholar] [CrossRef]
- Zhou, A.; Wu, H.; Pan, J.; Wang, X.; Li, J.; Wu, Z.; Hui, A. Synthesis and evaluation of paeonol derivatives as potential multifunctional agents for the treatment of Alzheimer’s disease. Molecules 2015, 20, 1304–1318. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.D.; Ghaskadbi, S.S. Protective effect of Pterostilbene against free radical mediated oxidative damage. BMC Complement Altern. Med. 2013, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Choi, S.H.; Gyawali, A.; Hyeon, S.J.; Kang, Y.S.; Ryu, H. Antioxidant and Neuroprotective Effects of Paeonol against Oxidative Stress and Altered Carrier-Mediated Transport System on NSC-34 Cell Lines. Antioxidants 2022, 11, 1392. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef] [PubMed]
- Roland, O.S.; Wang, J.; Wang, M.; Agbo, E.; Pang, D. The Antitumor Mechanism of Paeonol on CXCL4/CXCR3-B Signals in Breast Cancer Through Induction of Tumor Cell Apoptosis. Cancer Biother. Radiopharm. 2018, 33, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Zhou, Y.; Gao, Q.; Wang, R.; Fu, Y.; Zheng, L.; Yu, H. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxid. Med. Cell. Longev. 2020, 2020, 3469840. [Google Scholar] [CrossRef]
- Shi, H.; Hou, B.; Li, H.; Zhou, H.; Du, B. Cyclophosphamide Induces the Ferroptosis of Tumor Cells Through Heme Oxygenase-1. Front. Pharm. 2022, 13, 839464. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Pittalà, V.; Greish, K.; D’Amico, A.G.; Romeo, G.; Intagliata, S.; Salerno, L.; Vanella, L. Heme Oxygenase Modulation Drives Ferroptosis in TNBC Cells. Int. J. Mol. Sci. 2022, 23, 579. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z.; Xu, W.; Wang, Q.; Zhang, C.; Ding, Y.; Nie, W.; Lai, J.; Chen, Y.; Huang, H. Pterostilbene promotes mitochondrial apoptosis and inhibits proliferation in glioma cells. Sci. Rep. 2021, 11, 6381. [Google Scholar] [CrossRef]
- Moon, D.; McCormack, D.; McDonald, D.; McFadden, D. Pterostilbene induces mitochondrially derived apoptosis in breast cancer cells in vitro. J. Surg. Res. 2013, 180, 208–215. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Tsai, M.L.; Nagabhushanam, K.; Wang, Y.J.; Wu, C.H.; Ho, C.T.; Pan, M.H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consoli, V.; Sorrenti, V.; Burò, I.; Modica, M.N.; Vanella, L. Antiproliferative Effect of Plant-Derived Bioactive Compounds Endowed with Antioxidant Activity on Breast Cancer Cells. Nutraceuticals 2022, 2, 246-252. https://doi.org/10.3390/nutraceuticals2030018
Consoli V, Sorrenti V, Burò I, Modica MN, Vanella L. Antiproliferative Effect of Plant-Derived Bioactive Compounds Endowed with Antioxidant Activity on Breast Cancer Cells. Nutraceuticals. 2022; 2(3):246-252. https://doi.org/10.3390/nutraceuticals2030018
Chicago/Turabian StyleConsoli, Valeria, Valeria Sorrenti, Ilaria Burò, Maria N. Modica, and Luca Vanella. 2022. "Antiproliferative Effect of Plant-Derived Bioactive Compounds Endowed with Antioxidant Activity on Breast Cancer Cells" Nutraceuticals 2, no. 3: 246-252. https://doi.org/10.3390/nutraceuticals2030018
APA StyleConsoli, V., Sorrenti, V., Burò, I., Modica, M. N., & Vanella, L. (2022). Antiproliferative Effect of Plant-Derived Bioactive Compounds Endowed with Antioxidant Activity on Breast Cancer Cells. Nutraceuticals, 2(3), 246-252. https://doi.org/10.3390/nutraceuticals2030018







