Genotoxicity and Carcinogenicity of Medicinal Herbs and Their Nanoparticles
Abstract
:1. Introduction
1.1. Genotoxicity and Mutagenicity of Medicinal Plants
1.2. Clastogenicity and Carcinogenicity of Medicinal Plants
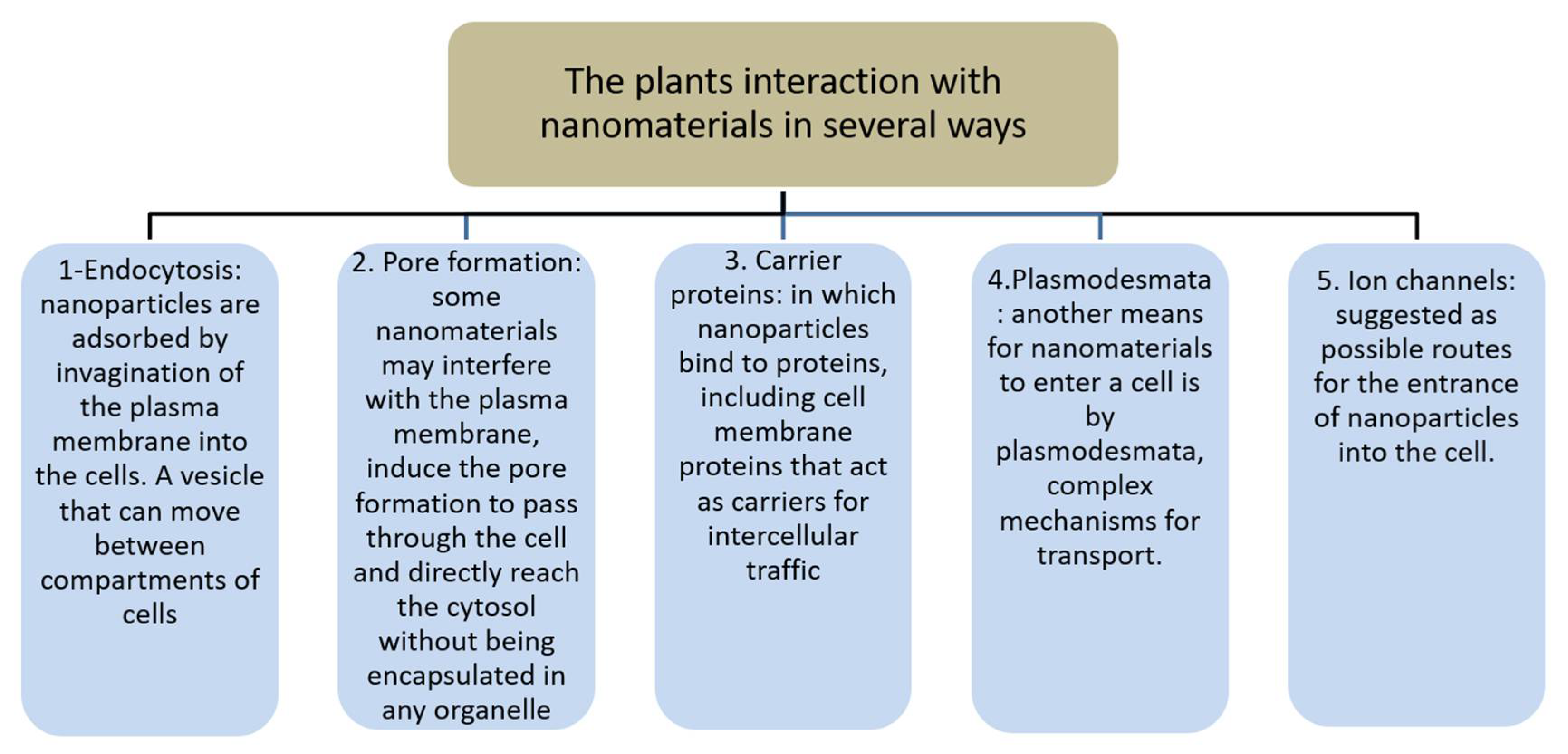
2. Nanoparticles from Plant Extracts
3. Nanomaterial Interaction with Tissues
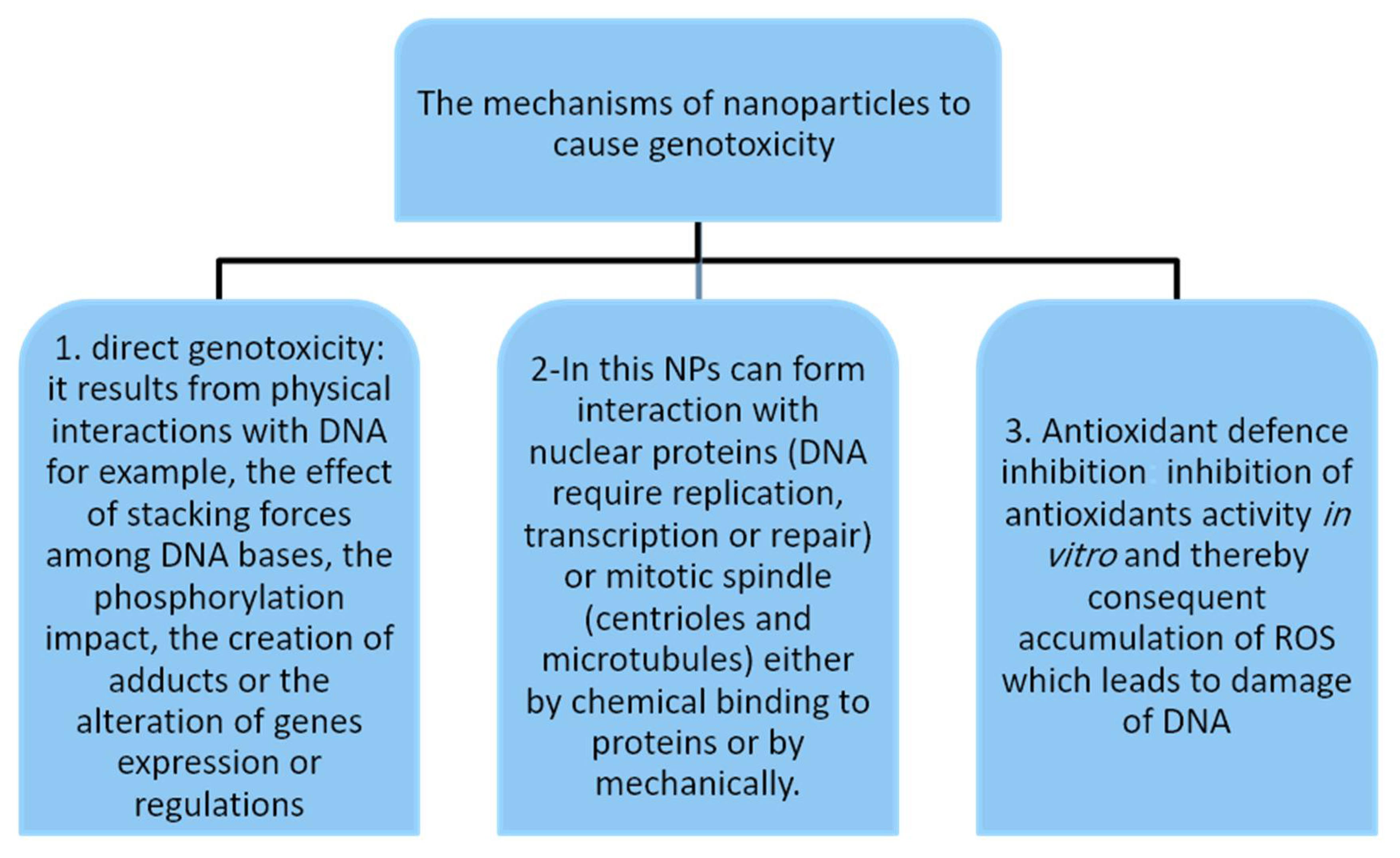
4. Mechanisms of Nanoparticle-Induced Genotoxicity
5. Carcinogenicity, Nanoparticles and Plants: A Significant Integration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poivre, M.; ENachtergael, A.; Bunel, V.; Philippe, O.; Duez, A. Genotoxicity and carcinogenicity of herbal products. In Toxicology of Herbal Products; Pelkonen, O., Duez, P., Vuorela, P.M., Vuorela, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 179–215. [Google Scholar]
- Zhou, J.; Ouedraogo, M.; Qu, F.; Duez, P. Potential genotoxicity of traditional Chinese medicinal plants and phytochemicals: An overview. Phytother. Res. 2013, 27, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Sponchiado, G.; Adam, M.L.; Silva, C.D.; Soley, B.S.; de Mello-Sampayo, C.; Cabrini, D.A.; Correr, C.J.; Otuki, M.F. Quantitative genotoxicity assays for analysis of medicinal plants: A systematic review. J. Ethnopharmacol. 2016, 178, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanan, M.; Asmalash, T.; Gebrekidan, A.; Gebreegziabiher, D.; Araya, T.; Hilekiros, H.; Barabadi, H.; Ramanathan, K. Nano-medicine as a newly emerging approach to combat human immunodeficiency virus (HIV). Pharm. Nanotechnol. 2018, 6, 17–27. [Google Scholar] [CrossRef]
- Barabadi, H.; Mahjoub, M.A.; Tajani, B.; Ahmadi, A.; Junejo, Y.; Saravanan, M. Emerging theranostic biogenic silver nanomaterials for breast cancer: A systematic review. J. Clust. Sci. 2019, 30, 259–279. [Google Scholar] [CrossRef]
- Wang, Y.P.; Yan, J.; Beger, R.D.; Fu, P.P.; Chou, M.W. Metabolic activation of the tumorigenic pyrrolizidine alkaloid, monocrotaline, leading to DNA adduct formation in vivo. Cancer Lett. 2005, 226, 27–35. [Google Scholar] [CrossRef]
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and characterization of biogenic tellurium nanoparticles by using Penicillium chrysogenum PTCC 5031: A novel approach in gold biotechnology. Iran. J. Pharm. Res. 2018, 17, 87–97. [Google Scholar]
- Barabadi, H. Nanobiotechnology: A promising scope of gold biotechnology. Cell. Mol. Biol. 2017, 63, 3–4. [Google Scholar] [CrossRef]
- Barabadi, H.; Honary, S.; Ebrahimi, P.; Alizadeh, A.; Naghibi, F.; Saravanan, M. Optimization of myco-synthesized silver nanoparticles by response surface methodology employing Box–Behnken design. Inorg. Nano-Met. Chem. 2019, 49, 33–43. [Google Scholar] [CrossRef]
- Salari, S.; Esmaeilzadeh Bahabadi, S.; Samzadeh-Kermani, A.; Yousefzaei, F. In vitro evaluation of antioxidant and antibacterial potential of green synthesized silver nanoparticles using Prosopis farcta fruit extract. Iran. J. Pharm. Res. 2019, 18, 430–445. [Google Scholar]
- Dobrucka, R. Synthesis of titanium dioxide nanoparticles using Echinacea purpurea herba. Iran. J. Pharm. Res. 2017, 16, 753–759. [Google Scholar]
- El-Rafie, H.M.; Zahran, M.K. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbo Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef]
- Tichy, J.; Novak, J. Extraction, assay, and analysis of antimicrobials from plants with activity against dental pathogens (Streptococcus sp.). J. Altern. Complement. Med. 1998, 4, 39–45. [Google Scholar] [CrossRef]
- Honary, S.; Barabadi, H.; Ebrahimi, P.; Naghibi, F.; Alizadeh, A. Development and optimization of biometal nanoparticles by using mathematical methodology: A microbial approach. J. Nano Res. 2015, 30, 106–115. [Google Scholar] [CrossRef]
- Rezvani Amin, Z.; Khashyarmanesh, Z.; Fazly Bazzaz, B.S.; SabetiNoghabi, Z. Does biosynthetic silver nanoparticles are more stable with lower toxicity than their synthetic counterparts? Iran. J. Pharm. Res. 2019, 18, 210–221. [Google Scholar] [PubMed]
- Barabadi, H.; Alizadeh, A.; Ovais, M.; Ahmadi, A.; Shinwari, Z.K.; Saravanan, M. Efficacy of green nanoparticles against cancerous and normal cell lines: A systematic review and meta-analysis. IET Nanobiotechnol. 2018, 12, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Didry, N.; Dubreuil, L.; Trotin, F.; Pinkas, M. Antimicrobial activity of aerial parts of Drosera peltata Smith on oral bacteria. J. Ethnopharmacol. 1998, 60, 91–96. [Google Scholar] [CrossRef]
- Benarba, B.; Pandiella, A. Medicinal plants as sources of active molecules against COVID-19. Front. Pharmacol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Cosyns, J.P.; Jadoul, M.; Squifflet, J.P.; Wese, F.X.; de Strihou, C.V.Y. Urothelial lesions in Chinese-herb nephropathy. Am. J. Kidney Dis. 1999, 33, 1011–1017. [Google Scholar] [CrossRef]
- Ernst, E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol. Sci. 2002, 23, 136–139. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Fard, J.K.; Hamzeiy, H.; Sattari, M.; Eftekhari, A.; Ahmadian, E.; Eghbal, M.A. Triazole rizatriptan induces liver toxicity through lysosomal/mitochondrial dysfunction. Drug Res. 2016, 66, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Babaei, H.; Nayebi, A.M.; Eftekhari, A.; Eghbal, M.A. Mechanistic approach for toxic effects of bupropion in primary rat hepatocytes. Drug Res. 2017, 67, 217–222. [Google Scholar] [CrossRef]
- Phillips, D.H.; Arlt, V.M. Genotoxicity: Damage to DNA and its consequences. In Molecular, Clinical and Environmental Toxicology; Springer: Cham, Switzerland, 2009; pp. 87–110. [Google Scholar]
- Mohamed, S.; Sabita, U.; Rajendra, S.; Raman, D. Genotoxicity: Mechanisms, testing guidelines and methods. Glob. J. Pharm. Sci. 2017, 1, 555–575. [Google Scholar]
- Caballero, B.; Trugo, L.C.; Finglas, P.M. Encyclopedia of Food Sciences and Nutrition; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Dhawan, A.; Shanker, R.; Kumar, A.; Dobrovolsky, V.N. Mutagenicity: Assays and Applications; Academic Press: London, UK, 2018. [Google Scholar]
- Kodym, A.; Afza, R. Physical and chemical mutagenesis. Plant Funct. Genom. 2015, 189–204. [Google Scholar] [CrossRef]
- IARC. Agents Classified by the IARC. Available online: https://monographs.iarc.fr/agents-classified-by-the-iarc/ (accessed on 16 November 2021).
- Biology Discussion. Types of Mutagens. 2016. Available online: Https://www.biologydiscussion.com/genetics/types-of-mutagens-chemical-and-physical-genetics/65297 (accessed on 16 November 2021).
- CDC. Facts about Sodium Azide. 2018. Available online: https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp (accessed on 22 May 2020).
- Olsen, O.; Wang, X.; Wettstein, D.V. Sodium azide mutagenesis: Preferential generation of A.T-->G.C transitions in the barley Ant18 gene. Proc. Natl. Acad. Sci. USA 1993, 90, 8043–8047. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Xia, Q.; Lin, G.; Chou, M. Genotoxic pyrrolizidine alkaloids–mechanisms leading to DNA adduct formation and tumorigenicity. Int. J. Mol. Sci. 2002, 3, 948–964. [Google Scholar] [CrossRef] [Green Version]
- Segelman, A.B.; Segelman, F.P.; Karliner, J.; Sofia, R.D. Sassafras and herb tea: Potential health hazards. JAMA 1976, 236, 477. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; MacLennan, R. Slaked lime and betel nut cancer in Papua New Guinea. Lancet 1992, 340, 577–578. [Google Scholar] [CrossRef]
- Chen, C.L.; Chi, C.W.; Chang, K.W.; Liu, T.Y. Safrole-like DNA adducts in oral tissue from oral cancer patients with a betel quid chewing history. Carcinogenesis 1999, 20, 2331–2334. [Google Scholar] [CrossRef] [Green Version]
- Barnes, J.; Anderson, L.; Phillipson, D.; Smith, M.; Veitch, N.C. Herbal Medicines; Pharmaceutical Press: London, UK, 2007. [Google Scholar]
- Hewitt, J.B. Effects of Occupational Exposure to Antineoplastic Drugs; University of Wisconsin: Madison, WI, USA, 1997. [Google Scholar]
- ILPI. Clastogen. 2020. Available online: http://www.ilpi.com/msds/ref/clastogen.html (accessed on 16 November 2021).
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; el Ghissassi, F.; Bouvard, V.; Altieri, A.; Cogliano, V. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007, 8, 292–293. [Google Scholar] [CrossRef]
- European Commission. Scientific Opinion on the Risk Assessment Methodologies and Approaches for Genotoxic and Carcinogenic Substances. 2009. Available online: https://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_113.pdf (accessed on 16 November 2021).
- Loeb, L. Mutator Phenotype. 1970. Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-642-16483-5_3913 (accessed on 7 June 2020).
- Debelle, F.D.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Nortier, J.; Pozdzik, A.; Roumeguere, T.; Vanherweghem, J.-L. Néphropathie aux Acidesaristolochiques (“Néphropathie aux Herbes Chinoises”). EMC—Néphrologie 2013, 10, 1–14. [Google Scholar] [CrossRef]
- Dictionary. 2020. Available online: Https://www.dictionary.com/browse/materials (accessed on 16 November 2021).
- Robertson, J.; Rizzello, L.; Avila-Olias, M.; Gaitzsch, J.; Contini, C.; Magon, M.; Renshaw, S.; Battaglia, G. Purification of nanoparticles by size and shape. Sci. Rep. 2016, 6, 27494. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in plant science: To make a long story short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Pérez-De-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Cruz, G.G.; Rodríguez-Fragoso, P.; Reyes-Esparza, J.; Rodríguez-López, A.; Gómez-Cansino, R.; Rodriguez-Fragoso, L. Interaction of nanoparticles with blood components and associated pathophysiological effects. In Unraveling the Safety Profile of Nanoscale Particles and Materials—From Biomedical to Environmental Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- Eivazzadeh-Keihan, R.; Maleki, A.; Guardia, M.D.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Alves, O.L.; Zucolotto, V.; Guterres, S. Nanotoxicology: Materials, Methodologies, and Assessments; Springer: New York, NY, USA, 2014. [Google Scholar]
- Mumtaz, A.; Mohammad, A.; Shahverdi, A.R. Role of natural products in green synthesis of nanoparticles. In Green Biosynthesis of Nanoparticles: Mechanisms and Applications; CABI: São Paulo, Brazil, 2013; pp. 31–52. [Google Scholar]
- Küünal, S.; Rauwel, P.; Rauwel, E. Plant extract mediated synthesis of nanoparticles. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; pp. 411–446. [Google Scholar]
- Mehrian, S.K.; Lima, R.D. Nanoparticles cyto and genotoxicity in plants: Mechanisms and abnormalities. Environ. Nanotechnol. Monit. Manag. 2016, 6, 184–193. [Google Scholar]
- Burdușel, A.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Patlolla, A.K.; Tchounwou, P.B. Genotoxicity of silver nanoparticles (Ag-NPs) in in vitro and in vivo models. In Nanotechnology in Skin, Soft Tissue, and Bone Infections; Springer: Cham, Switzerland, 2020; pp. 269–281. [Google Scholar]
- Patra, C.R.; Mukherjee, S.; Kotcherlakota, R. Biosynthesized silver nanoparticles: A step forward for cancer theranostics? Nanomedicine 2014, 9, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934. [Google Scholar]
- Klefenz, H. Nanobiotechnology: From molecules to systems. Eng. Life Sci. 2004, 4, 211–218. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Bhatte, K.D.; Deshmukh, K.M.; Patil, Y.P.; Sawant, D.N.; Fujita, S.-I.; Arai, M.; Bhanage, B.M. Synthesis of powdered silver nanoparticles using hydrogen in aqueous medium. Particuology 2012, 10, 140–143. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.; Gaikwad, S. Fungi: Myconanofactory, mycoremediation and medicine. In Fungi: Applications and Management Strategies; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Shankar, S.S.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Biological synthesis of triangular gold nanoprisms. Nat. Mater. 2004, 3, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Rath, M.; Panda, S.S.; Dhal, N.K. Synthesis of silver nano particles from plant extract and its application in cancer treatment: A review. Int. J. Plant Anim. Environ. Sci. 2016, 4, 137–145. [Google Scholar]
- EMEA. Guideline on the Assessment of Genotoxicity of Herbal Substances/Preparations. 2008. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003569.pdf (accessed on 16 November 2021).
- SFDA. Saudi FDA Products Classification Guidance. 2020. Available online: https://old.sfda.gov.sa/ar/oper/Documents/SFDAProductsClassificationGuidance.pdf (accessed on 16 November 2021).
| Chemical | Carcinogenicity |
|---|---|
| EMS | + |
| HN2 | + |
| DES | + |
| HMPA | + |
| Benzene | + |
| EO | +/− |
| DMF | − |
| DMN | + |
| 4CMB | − |
| IsoPC | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qari, S.H.; Alrefaei, A.F.; Ashoor, A.B.; Soliman, M.H. Genotoxicity and Carcinogenicity of Medicinal Herbs and Their Nanoparticles. Nutraceuticals 2021, 1, 31-41. https://doi.org/10.3390/nutraceuticals1010005
Qari SH, Alrefaei AF, Ashoor AB, Soliman MH. Genotoxicity and Carcinogenicity of Medicinal Herbs and Their Nanoparticles. Nutraceuticals. 2021; 1(1):31-41. https://doi.org/10.3390/nutraceuticals1010005
Chicago/Turabian StyleQari, Sameer H., Abdulmajeed F. Alrefaei, Ahmed B. Ashoor, and Mona H. Soliman. 2021. "Genotoxicity and Carcinogenicity of Medicinal Herbs and Their Nanoparticles" Nutraceuticals 1, no. 1: 31-41. https://doi.org/10.3390/nutraceuticals1010005







