Filamentous Fungi in Drinking Water, Particularly in Relation to Biofilm Formation
Abstract
:1. Introduction
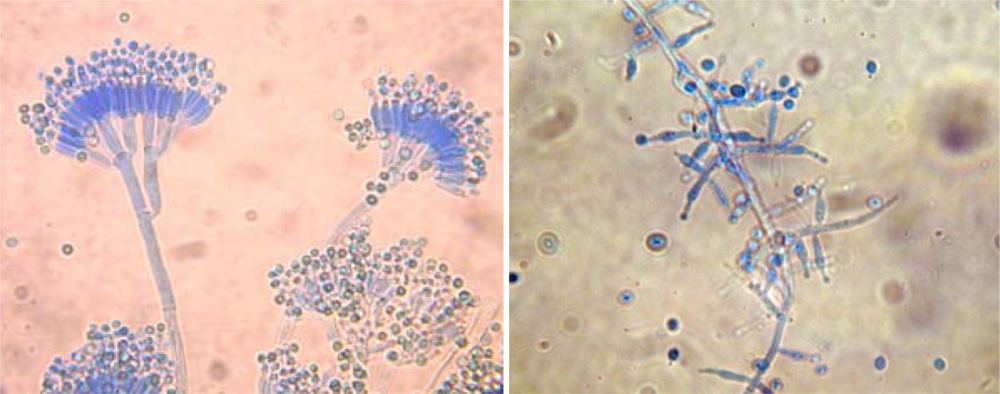
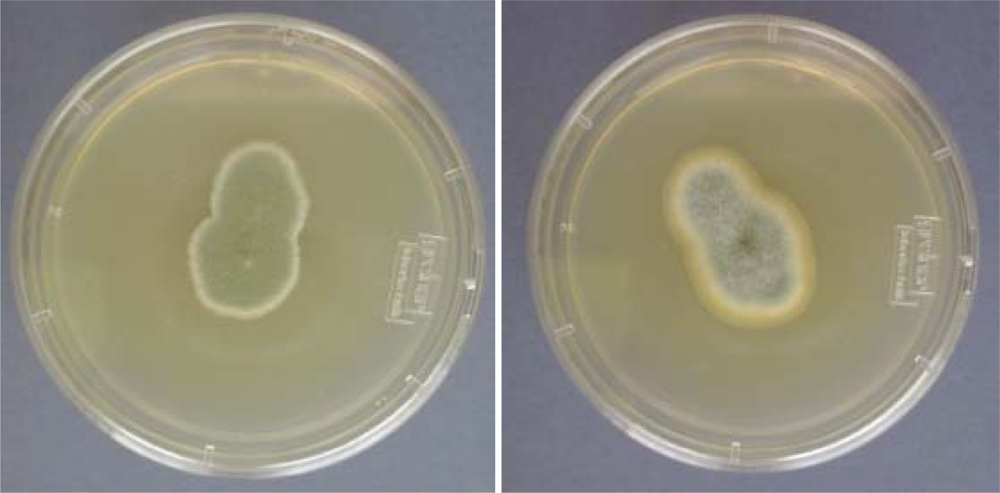
2. Methods to Study Fungi in Tap Water
3. Fungal Water Biofilms
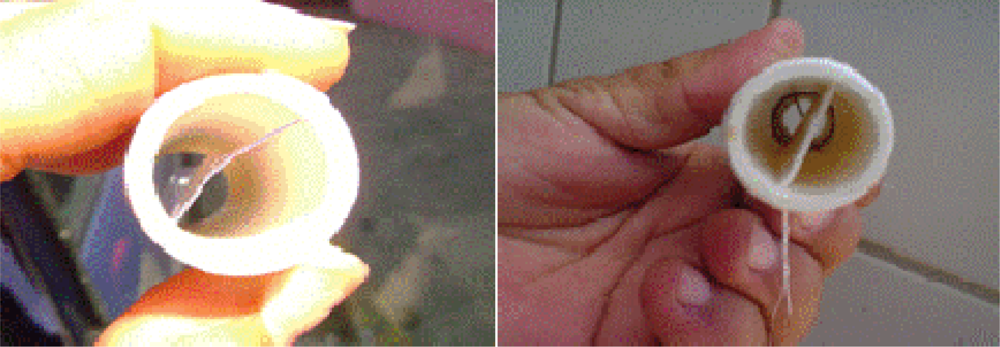
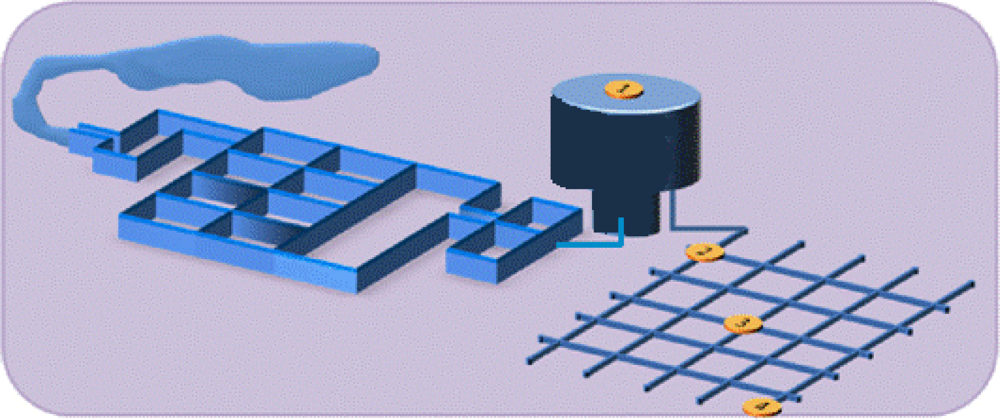
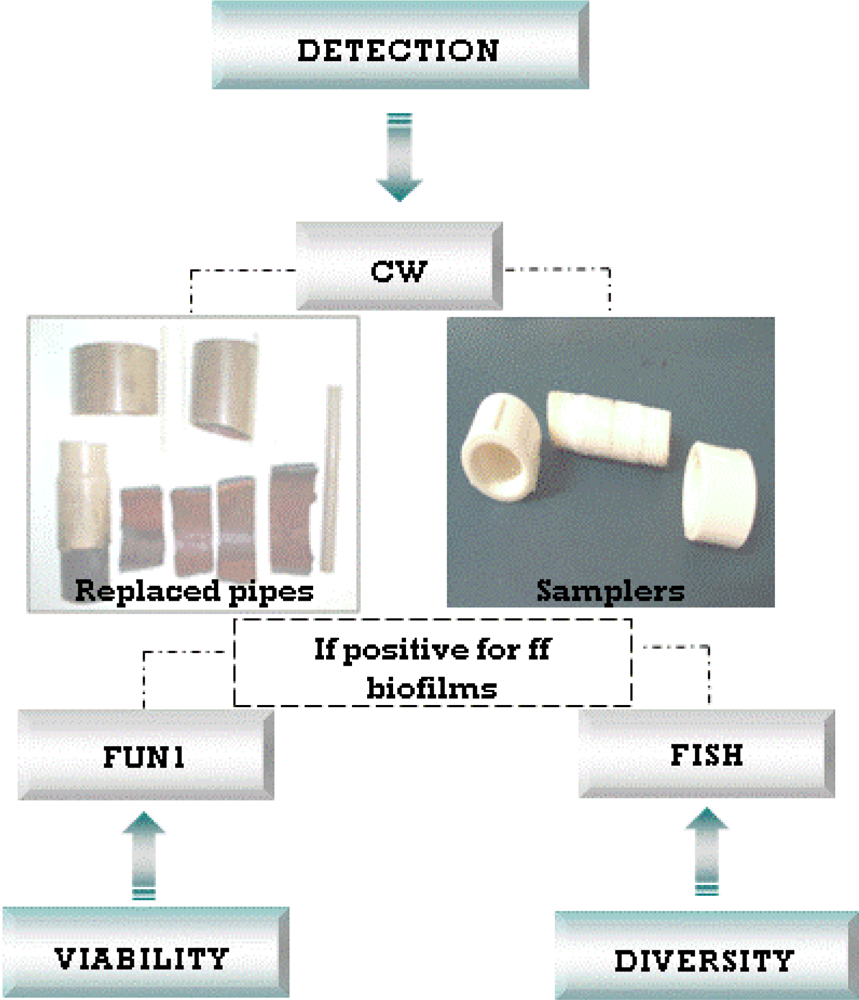
3.1. Sampling Biofilms
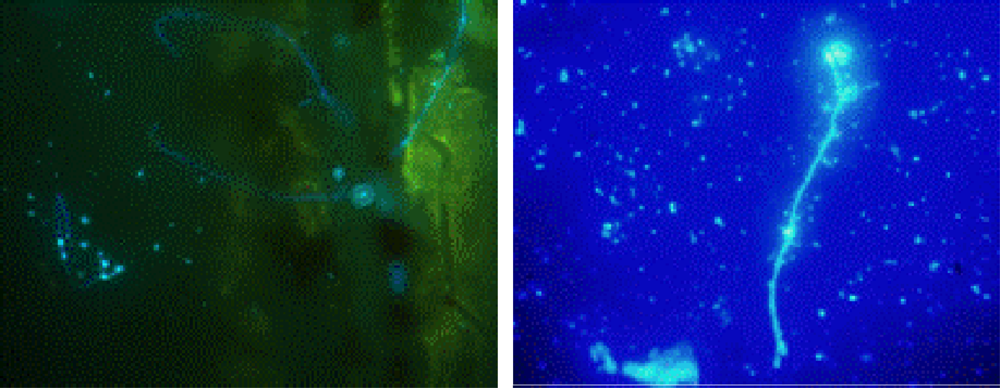
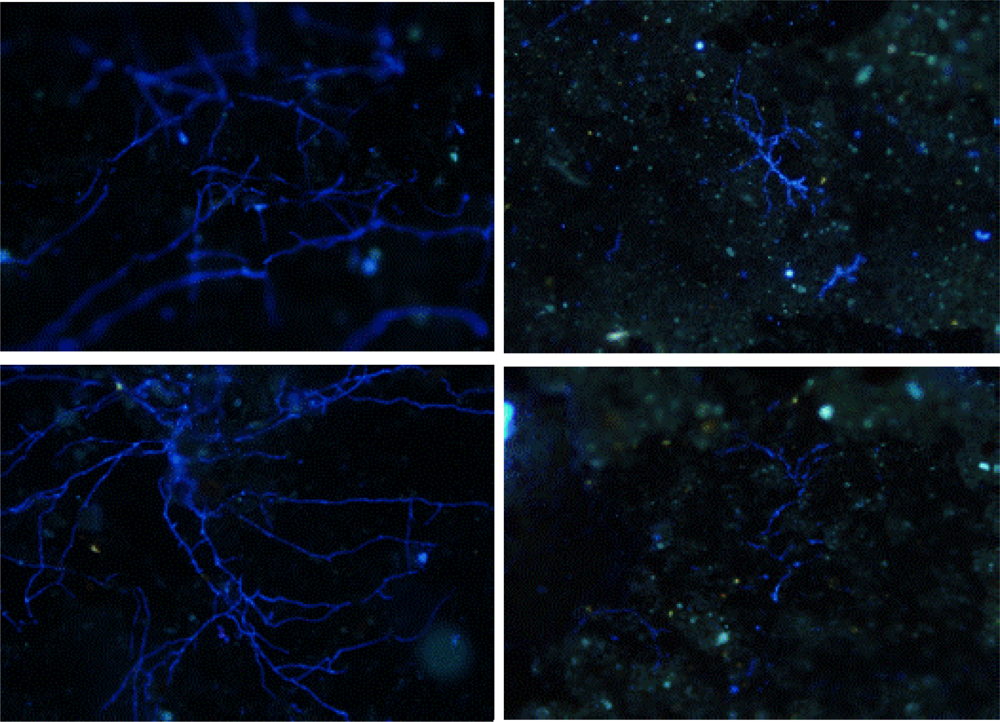
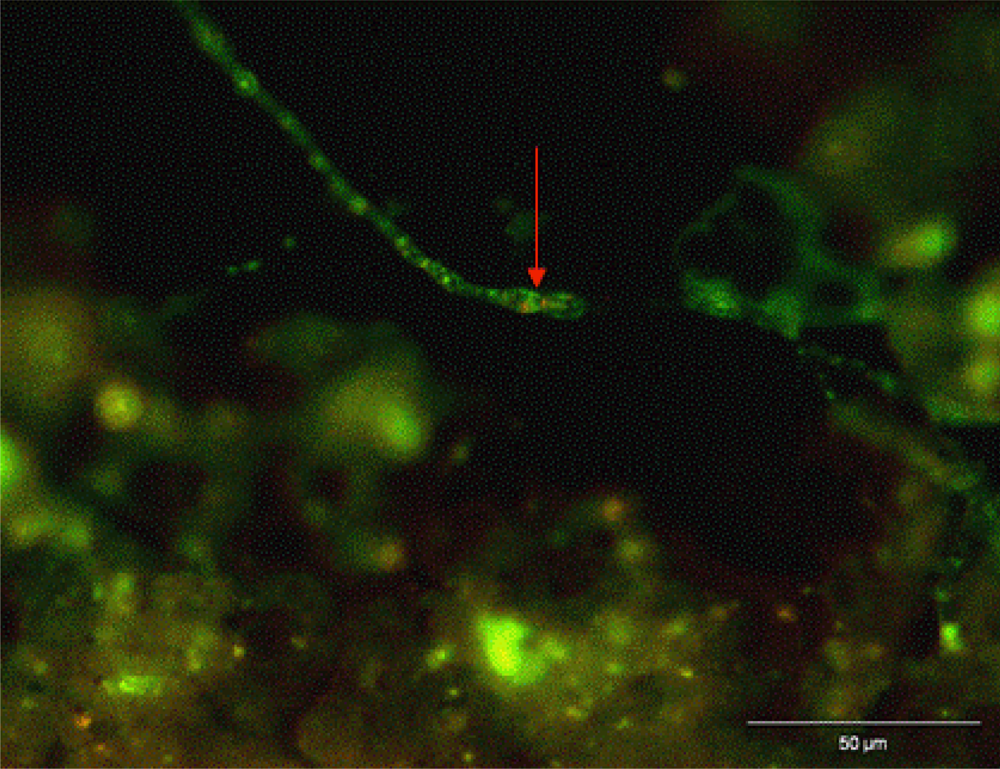
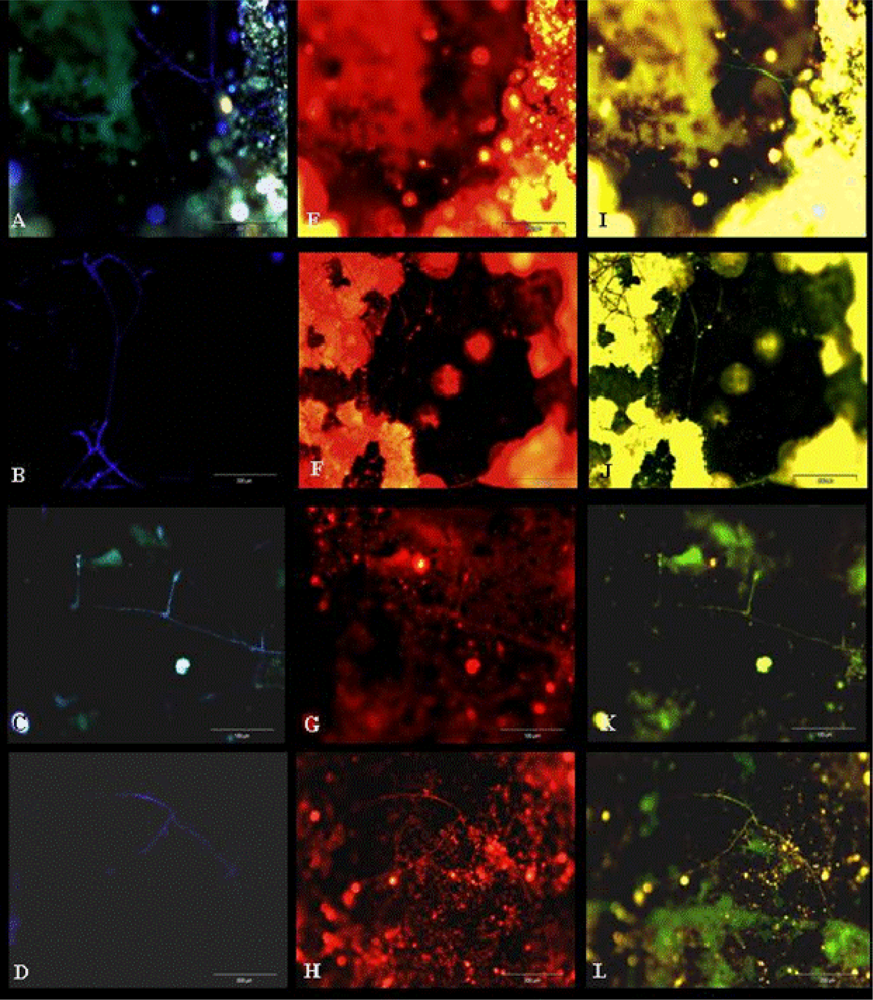
3.2. Biofilm Detection
4. Conclusions and Recommended Procedures
Acknowledgments
References
- Nagy, LA; Olson, BH. The occurrence of filamentous fungi in drinking water distribution systems. Can. J. Microbiol 1982, 28, 667–671. [Google Scholar]
- Nagy, LA; Olson, BH. Occurrence and significance of bacteria, fungi and yeasts associated with distribution pipe surfaces. Proceedings of the American Water Works Association, Water Quality Technology Conference, Houston, TX, December 1985; American Water Works Association: Denver, CO, USA, 1985; pp. 213–238. [Google Scholar]
- Franková, E; Horecka, M. Filamentous soil fungi and unidentified bacteria in drinking water from wells and water mains near Bratislava. Microbiol. Res 1995, 150, 311–313. [Google Scholar]
- Kinsey, GC; Paterson, RR; Kelley, J. Methods for the determination of filamentous fungi in treated and untreated waters. J. Appl. Microbiol. Symp. Suppl 1999, 85, 214S–224S. [Google Scholar]
- Göttlich, E; van der Lubbe, W; Lange, B; Fiedler, S; Melchert, I; Reifenrath, M; Flemming, H-C; Hoog, S. Fungal flora in groundwater-derived public drinking water. Int. J. Hyg. Environ. Health 2002, 205, 269–279. [Google Scholar]
- Kelley, J; Kinsey, G; Paterson, R; Brayford, D; Pitchers, R; Rossmore, H. Identification and Control of Fungi in Distribution Systems; AWWA Research Foundation and American Water Works Association: Denver, CO, USA, 2003. [Google Scholar]
- Paterson, RRM; Lima, N. Fungal Contamination of Drinking Water. In Water Encyclopedia; Lehr, J, Keeley, J, Lehr, J, Kingery, TB, III, Eds.; John Wiley & Sons: New York, NY, USA, 2005; pp. 1–7. [Google Scholar]
- Gonçalves, AB; Paterson, RRM; Lima, N. Survey and significance of filamentous fungi from tap water. Int. J. Hyg. Environ. Health 2006, 209, 257–264. [Google Scholar] [Green Version]
- Hageskal, G; Knutsen, AK; Gaustad, P; de Hoog, GS; Skaar, I. Diversity and significance of mold species in Norwegian drinking water. Appl. Environ. Microbiol 2006, 72, 7586–7593. [Google Scholar]
- Hageskal, G; Gaustad, P; Heier, BT; Skaar, I. Occurrence of moulds in drinking water. J. Appl. Microbiol 2007, 102, 774–780. [Google Scholar]
- Hageskal, G; Lima, N; Skaar, I. The study of fungi in drinking water. Mycol. Res 2009, 133, 165–172. [Google Scholar]
- Paterson, RRM; Hageskal, G; Skaar, I; Lima, N. Occurrence, problems, analysis and removal of filamentous fungi in drinking water. In Fungicides: Chemistry, Environmental Impact and Health Effects; De Costa, P, Bezerra, P, Eds.; Nova Science Publishers: New York, NY, USA, 2009; pp. 379–399. [Google Scholar]
- Sammon, NB; Harrower, KM; Fabbro, LD; Reed, RH. Incidence and distribution of microfungi in a treated 9 municipal water supply system in sub-tropical Australia. Int. J. Environ. Res. Public Health 2010, 7, 1597–1611. [Google Scholar]
- Arvanitidou, M; Kanellou, K; Constantinides, TC; Katsouyannopoulos, V. The occurrence of fungi in hospital and community potable waters. Lett. Appl. Microbiol 1999, 29, 81–84. [Google Scholar]
- Arvanitidou, M; Spaia, S; Velegraki, A; Pezarloglou, M; Kanetidis, D; Pangidis, P; Askepidis, N; Katsinas, Ch; Vayonas, G; Katsouyannopoulos, V. High level of recovery of fungi from water and dialysate in haemodialysis units. J. Hosp. Infect 2000, 45, 225–230. [Google Scholar]
- Nazim, S; Dawar, S; Tariq, M; Zaki, MJ. Quantitative estimation of mycoflora in drinking water and fruit juices of Karachi. Pakistan J. Bot 2008, 40, 1263–1268. [Google Scholar]
- Oliveira, HMB. Fungos filamentosos na água e em biofilmes na rede de distribuição de água potável do sistema Alto do Céu, Recife-PE, Masters Thesis, Biologia de Fungos do Departamento de Micologia do Centro de Ciências Biológicas da Universidade Federal de Pernambuco (UFPE), Recife, Brazil. 2010.
- Pereira, VJ; Basílio, MC; Fernandes, D; Domingues, M; Paiva, JM; Benoliel, MJ; Crespo, MT; San Romão, MV. Occurrence of filamentous fungi and yeasts in three different drinking water sources. Water Res 2009, 43, 3813–3819. [Google Scholar]
- Pereira, VJ; Fernandes, D; Carvalho, G; Benoliel, MJ; San Romão, MV; Barreto Crespo, MT. Assessment of the presence and dynamics of fungi in drinking water sources using cultural and molecular methods. Water Res 2010, 44, 4850–4859. [Google Scholar]
- Santos, C; Paterson, RRM; Venâncio, A; Lima, N. Filamentous fungal characterisations by matrix-assisted laser desorption/ionisation time of flight mass spectrometry. J. Appl. Microbiol 2010, 8, 375–385. [Google Scholar]
- Santos, C; Fraga, ME; Kozakiewicz, Z; Lima, N. Fourier transform infrared as a powerful technique for the identification and characterisation of filamentous fungi and yeasts. Res. Microbiol 2010, 161, 168–175. [Google Scholar]
- Huq, A; Whitehouse, CA; Grim, CJ; Alam, M; Colwell, RR. Biofilms in water, its role and impact in human disease transmission. Curr. Opin. Biotechnol 2008, 19, 244–247. [Google Scholar]
- Boe-Hansen, R; Martiny, AC; Arvin, E; Albrechtsen, H-J. Monitoring biofilm formation and activity in drinking water distribution networks under oligotrophic conditions. Water Sci. Technol 2003, 47, 91–97. [Google Scholar]
- Niquette, P; Servais, P; Savoir, R. Impacts of pipe materials on densities of fixed bacterial biomass in a drinking water distribution system. Water Res 2000, 34, 1952–1956. [Google Scholar]
- McCoy, WF; Bryers, JD; Robbins, J; Costerton, JW. Observations of fouling biofilm formation. Can. J. Microbiol 1981, 27, 910–917. [Google Scholar]
- Van der Wende, E; Characklis, WG; Smith, DB. Biofilms and bacterial drinking water quality. Water Res 1989, 3, 1313–1322. [Google Scholar]
- Caldwell, DE; Lawrence, JR. Study of attached cells in continues-flow slide culture. In CRC Handbook of Laboratory Systems for Microbial Ecology Research; Wimpenny, JWT, Ed.; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Li, Y; Dick, WA; Tuovinen, OH. Fluorescence microscopy for visualization of soil microorganisms—a review. Biol. Fert. Soils 2004, 39, 301–311. [Google Scholar]
- Gonçalves, AB; Santos, IM; Paterson, RRM; Lima, N. FISH and Calcofluor staining techniques to detect in situ filamentous fungal biofilms in water. Revista Iberoamericana de Micologia 2006, 23, 192–196. [Google Scholar]
- Determination with the membrane filters method, NS 4716. In Norwegian Standard for Water Analysis: Microfungi in Water; The Standardization Organizations in Norway: Oslo, Norway, 1991; pp. 1–12. (In Norwegian)
| Country, Place, Year | Period of time | Type of water | Main isolation method | Most frequent fungal isolates | Refs. |
|---|---|---|---|---|---|
| United Kingdom, 1996 | Autumn and Spring | Surface water and network | Membrane filtration, Direct plating and Bating | Aspergillus, Cladosporium, Epicoccum, Penicillium and Trichoderma | [4] |
| Greece, Thessaloniki, 1998 | One collection (126 samples) | Tap water (hospital and community) | Membrane filtration | Penicillium, Aspergillus and Acremonium | [14] |
| Greece, 85 haemodialysis units, 1998 | One collection (255 samples) | Municipal water supplies of haemodialysis centres | Membrane filtration | Penicillium and Aspergillus | [15] |
| Germany, North Rhine-Westphalia, 1998/9 | 12 months | Drinking water | Pour-plating | Acremonium, Exophiala, Penicillium and Phialophora | [5] |
| Norway, 14 networks, 2002/3 | December, June and September | Drinking water (surface and groundwater) | Membrane filtration | Penicillium, Trichoderma and Aspergillus | [9,10] |
| Portugal, Braga, 2003/4 | 12 months | Tap water | Membrane filtration | Penicillium and Acremonium | [8] |
| Pakistan, Karachi, 2007 | One collection (30 samples) | Water (and fruit juice) | Direct plating | Aspergillus niger and A. clavatus | [16] |
| Australia, Queensland, 2007/8 | 18 months | Municipal water | Membrane filtration | Cladosporium, Penicillium, Aspergillus and Fusarium | [13] |
| Brazil, Recife, 2009/10 | 5 months | Water treatment plant; tap water | Membrane filtration | Penicillium, Aspergillus and Phoma | [17] |
| Portugal, Lisbon, 2010 | 4 months | surface water; spring water; groundwater | Membrane filtration | Aspergillus, Cladosporium, Penicillium | [18,19] |
| Medium | Composition | |
|---|---|---|
| CMA/2—Corn meal agar half-strength | Corn meal | 8.5 g |
| Agar | 8.5 g | |
| Distilled water | 1000 mL | |
| pH | 5.8–6.2 | |
| CZ—Czapek Dox agar | Czapek solution | 10 mL |
| K2HPO4 | 1 g | |
| Saccharose | 30 g | |
| Agar | 15 g | |
| Distilled water | 1000 mL | |
| pH | 6.0–6.4 | |
| Czapek solution | ||
| NaNO3 | 30 g | |
| KCl | 5 g | |
| MgSO4·7H2O | 5 g | |
| FeSO4·7H2O | 0.1 g | |
| ZnSO4·7H2O | 0.1 g | |
| CuSO4·5H2O | 0.05 g | |
| Distilled water | 100 mL | |
| DG18—Dichloran 18 % glycerol agar | Peptone | 5 g |
| Glucose | 10 g | |
| K2HPO4 | 1 g | |
| MgSO4·7H2O | 0.5 g | |
| Dichloran (0.2% in ethanol) | 1 mL | |
| Glycerol | 220 g | |
| Chloramphenicol | 0.1 g | |
| Agar | 15 g | |
| Distilled water | 1000 mL | |
| pH | 5.4–5.8 | |
| DRBC—Dichloran Rose Bengal chloramphenicol agar | Peptone | 5 g |
| Glucose | 10 g | |
| K2HPO4 | 1 g | |
| MgSO4·7H2O | 0.5 g | |
| Dichloran | 0.002 g | |
| Rose Bengal | 0.025 g | |
| Chloramphenicol | 0.1 g | |
| Agar | 15 g | |
| Distilled water | 1000 mL | |
| pH | 5.4–5.8 | |
| MEA—Malt extract agar | Malt extract | 20 g |
| Peptone | 1 g | |
| Glucose | 20 g | |
| Agar | 20 g | |
| Distilled water | 1000 mL | |
| pH | 5.0–5.5 | |
| NGRBA—Neopeptone glucose Rose Bengal aureomycin | Neopeptone | 5 g |
| Glucose | 10 g | |
| 0.67 % (w/v) aureomycin solution | 5 mL | |
| 1 % (w/v) Rose Bengal solution | 3.5 mL | |
| Agar | 15 g | |
| Distilled water | 1000 mL | |
| pH | 6.3–6.7 | |
| PDA—Potato dextrose agar | Potato extract | 4 g |
| Glucose | 20 g | |
| Agar | 15 g | |
| Distilled water | 1000 mL | |
| pH | 5.4–5.8 | |
| SDA—Sabouraud dextrose agar | Mycological peptone | 10 g |
| Glucose | 40 g | |
| Agar | 15 g | |
| Distilled water | 1000 mL | |
| pH | 5.4–5.8 | |


© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Siqueira, V.M.; Oliveira, H.M.B.; Santos, C.; Paterson, R.R.M.; Gusmão, N.B.; Lima, N. Filamentous Fungi in Drinking Water, Particularly in Relation to Biofilm Formation. Int. J. Environ. Res. Public Health 2011, 8, 456-469. https://doi.org/10.3390/ijerph8020456
Siqueira VM, Oliveira HMB, Santos C, Paterson RRM, Gusmão NB, Lima N. Filamentous Fungi in Drinking Water, Particularly in Relation to Biofilm Formation. International Journal of Environmental Research and Public Health. 2011; 8(2):456-469. https://doi.org/10.3390/ijerph8020456
Chicago/Turabian StyleSiqueira, Virgínia M., Helena M. B. Oliveira, Cledir Santos, R. Russell M. Paterson, Norma B. Gusmão, and Nelson Lima. 2011. "Filamentous Fungi in Drinking Water, Particularly in Relation to Biofilm Formation" International Journal of Environmental Research and Public Health 8, no. 2: 456-469. https://doi.org/10.3390/ijerph8020456
APA StyleSiqueira, V. M., Oliveira, H. M. B., Santos, C., Paterson, R. R. M., Gusmão, N. B., & Lima, N. (2011). Filamentous Fungi in Drinking Water, Particularly in Relation to Biofilm Formation. International Journal of Environmental Research and Public Health, 8(2), 456-469. https://doi.org/10.3390/ijerph8020456







