Improving Breast Cancer Outcomes Through Quality Care: Call to Action for the Implementation of the Breast Cancer Care Quality Index (BCCQI)
Highlights
- Breast cancer is the most common cancer among women globally, with increasing incidence, substantial mortality, and economic burden.
- Breast cancer has wide-ranging impacts, affecting individuals’ mental and physical health, disrupting household stability, productivity, equity, and social well-being, and placing strain on healthcare systems.
- Most countries are not on track to achieve global targets for reducing breast cancer mortality, underscoring major gaps in care quality and access, and highlighting the urgency of addressing these issues.
- This paper supports the implementation of the Breast Cancer Care Quality Index, a practical framework to address persistent global inequalities through the assessment and improvement of breast cancer care quality.
- Countries can leverage this Call to Action to prioritize context-appropriate interventions through a structured, tiered self-assessment approach applicable across the breast cancer care continuum, including early detection, timely diagnosis, comprehensive management, and broader healthcare system components.
- By aligning actions around essential care elements, the Call to Action helps stakeholders identify priorities, foster coordination, and develop actionable roadmaps that translate commitments into measurable improvements and high-quality care for all women.
Abstract
1. Breast Cancer: An Unfinished Agenda
1.1. Background
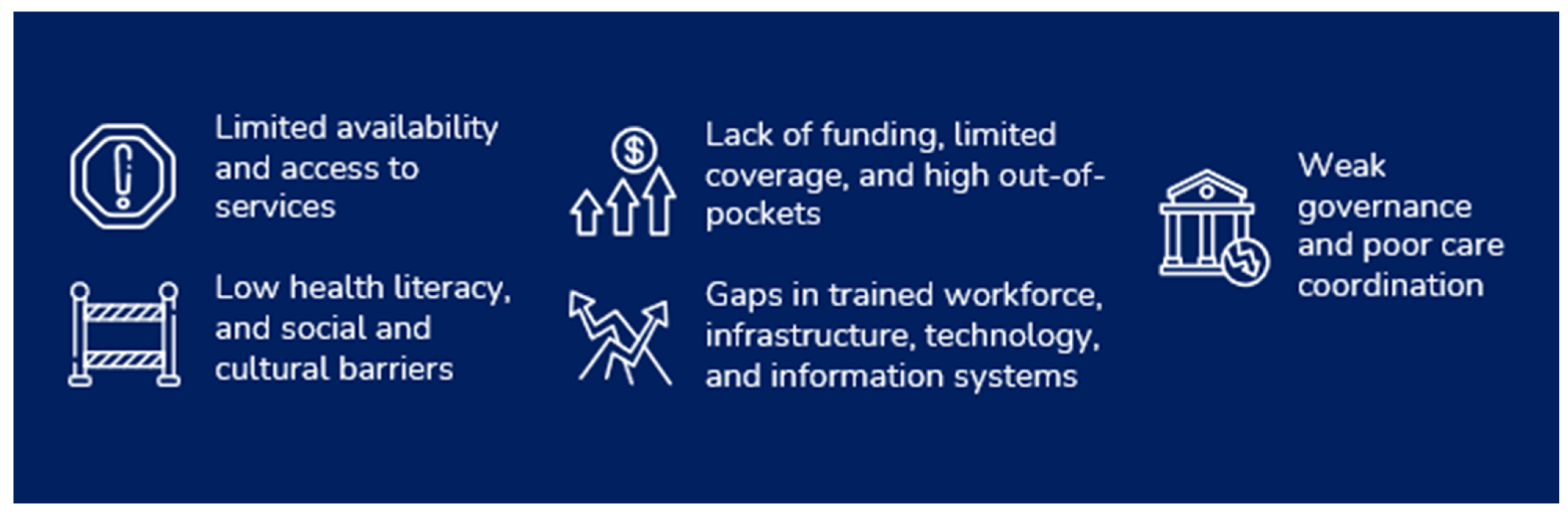
1.2. Challenges in Breast Cancer Care
2. Need for Action
3. Introducing the Breast Cancer Care Quality Index (BCCQI)
4. Country Profiling Using the Breast Cancer Care Quality Index (BCCQI)
5. Strategic Recommendations: Scaling up the Breast Cancer Care Quality Index (BCCQI)
- Meet the Breast Cancer Care Quality Index (BCCQI) targets;
- Improve performance on key indicators;
- Align stakeholders at both national and international levels around common priorities.
- Drafted or recently adopted national policy or framework, outlining basic measures for early detection.
- Awareness and education programs exist but are fragmented or pilot-based, not integrated into national health frameworks.
- Some primary health or community-level services attempt early identification and referral, but pathways have not been formally established.
- No definition of women at elevated risk and targeted screening limited to certain jurisdictions, or dependent on external funding.
- <25% of invasive breast cancers diagnosed at stage I or II according to TNM anatomical or pathological staging or no data for this indicator.
- Develop risk-assessment tools: With the support of national or international stakeholders, develop context-specific tools for individual risk assessment to be administered to women during GP visits.
- Establish referral pathways: Pilot basic referral pathways for suspicious findings in high-population areas as a model for future scale-up to reach rural or marginalized jurisdictions.
- Promote implementation of breast cancer policy: Advocate for stepwise institutionalization of measures from the proposed or recently adopted breast cancer detection plan.
- Develop a definition of women at elevated risk of breast cancer: Develop an evidence-based definition of women at elevated risk of breast cancer to delineate the target population for the national early detection strategy and prioritize higher-frequency screening for this group.
- Develop a definition of women at elevated risk of breast cancer: Develop an evidence-based definition of women at elevated risk of breast cancer to delineate the target population for the national early detection strategy and prioritize higher-frequency screening for this group.
- Standardize risk-assessment in primary healthcare: Develop context-specific tools for individual risk-assessment to be administered to women during GP visits and establish national breast cancer risk-assessment protocols to be rolled out to GPs at the national level.
- Expand public and workforce awareness: Strengthen health promotion efforts, such as early detection awareness campaigns for the public, and scale education programs for healthcare workers by integrating breast cancer modules into existing national training platforms.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Breast Cancer Initiative Implementation Framework: Assessing, Strengthening, and Scaling Up of Services for the Early Detection and Management of Breast Cancer; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- World Health Organization. Breast Cancer. Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 18 August 2025).
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Cancer Today- Age-Standardized Rate (World) per 100,000, Incidence and Mortality, Both Sexes, in 2022. Available online: https://gco.iarc.fr/today/en/dataviz/bars?types=0_1&mode=population&cancers=20&sort_by=value1&populations=903_904_905_908_909_935 (accessed on 4 February 2025).
- Coles, C.E.; Earl, H.; Anderson, B.O.; Barrios, C.H.; Bienz, M.; Bliss, J.M.; Cameron, D.A.; Cardoso, F.; Cui, W.; Francis, P.A.; et al. The Lancet Breast Cancer Commission. Lancet 2024, 403, 1895–1950. [Google Scholar] [CrossRef]
- Anderson, B.O.; Ilbawi, A.M.; Fidarova, E.; Weiderpass, E.; Stevens, L.; Abdel-Wahab, M.; Mikkelsen, B. The Global Breast Cancer Initiative: A Strategic Collaboration to Strengthen Health Care for Non-Communicable Diseases. Lancet Oncol. 2021, 22, 578–581. [Google Scholar] [CrossRef]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global Patterns and Trends in Breast Cancer Incidence and Mortality across 185 Countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Olopade, O.I. A Perfect Storm: How Tumor Biology, Genomics, and Health Care Delivery Patterns Collide to Create a Racial Survival Disparity in Breast Cancer and Proposed Interventions for Change. CA Cancer J. Clin. 2015, 65, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Addai, B.W.; Adewole, I.; Ainsworth, V.; Alaro, J.; Alatise, O.I.; Ali, Z.; Anderson, B.O.; Anorlu, R.; Avery, S.; et al. Cancer in Sub-Saharan Africa: A Lancet Oncology Commission. Lancet Oncol. 2022, 23, e251–e312. [Google Scholar] [CrossRef] [PubMed]
- Brand, N.R.; Qu, L.G.; Chao, A.; Ilbawi, A.M. Delays and Barriers to Cancer Care in Low- and Middle-Income Countries: A Systematic Review. Oncologist 2019, 24, e1371–e1380. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, C.I. Racial Disparities in Breast Cancer Diagnosis and Treatment by Hormone Receptor and HER2 Status. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Trapani, D.; Ginsburg, O.; Fadelu, T.; Lin, N.U.; Hassett, M.; Ilbawi, A.M.; Anderson, B.O.; Curigliano, G. Global Challenges and Policy Solutions in Breast Cancer Control. Cancer Treat. Rev. 2022, 104, 102339. [Google Scholar] [CrossRef] [PubMed]
- Ghebreyesus, T.A.; Mired, D.; Sullivan, R.; Mueller, A.; Charalambous, A.; Kacharian, A.; Tsagkaris, C.; Soto-Perez-de-Celis, E.; Grigoryan, H.; Gralow, J.; et al. A Manifesto on Improving Cancer Care in Conflict-Impacted Populations. Lancet 2024, 404, 427. [Google Scholar] [CrossRef]
- eClinicalMedicine. Breast Cancer–the Impact of Conflict and Displacement. EClinicalMedicine 2024, 76, 102906. [Google Scholar] [CrossRef] [PubMed]
- Essue, B.M.; Danforth, K.; Langer, A.; Acharya, P.; Knaul, F.M. The Economics of Investing in Women and Health. Nat. Med. 2025, 31, 2532–2545. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef]
- Liao, L. Inequality in Breast Cancer: Global Statistics from 2022 to 2050. Breast 2025, 79, 103851. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, Y.; Liu, S.; Li, J.; Wu, J.; Jin, Q.; Liu, X.; Duan, H.; Feng, Z.; Liu, Y.; et al. Global Burden of Female Breast Cancer: New Estimates in 2022, Temporal Trend and Future Projections up to 2050 Based on the Latest Release from GLOBOCAN. J. Natl. Cancer Cent. 2025, 5, 287–296. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals. 2023. Available online: https://sdgs.un.org/goals (accessed on 28 September 2024).
- Cazap, E.; Anderson, B.O.; Curigliano, G.; Sehdev, S.; Cardoso, F.; Gonzalez, A.R.; Shash, E.; Yip, C.-H.; Mattar, A.; Chavarri-Guerra, Y.; et al. Bridging Gaps in Breast Cancer Care: A Breast Cancer Care Quality Index to Improve Outcomes Worldwide. Ecancermedicalscience 2025, 19, 1981. [Google Scholar] [CrossRef]
- World Health Organization. Cancer—Screening and Early Detection. Available online: https://www.who.int/europe/news-room/fact-sheets/item/cancer-screening-and-early-detection-of-cancer (accessed on 13 May 2024).
- Srinath, A.; van Merode, F.; Rao, S.V.; Pavlova, M. Barriers to Cervical Cancer and Breast Cancer Screening Uptake in Low- and Middle-Income Countries: A Systematic Review. Health Policy Plan. 2023, 38, 509–527. [Google Scholar] [CrossRef]
- Getachew, S.; Tesfaw, A.; Kaba, M.; Wienke, A.; Taylor, L.; Kantelhardt, E.J.; Addissie, A. Perceived Barriers to Early Diagnosis of Breast Cancer in South and Southwestern Ethiopia: A Qualitative Study. BMC Womens Health 2020, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Devi, G.R.; Fish, L.J.; Bennion, A.; Sawin, G.E.; Weaver, S.M.; Reddy, K.; Saincher, R.; Tran, A.N. Identification of Barriers at the Primary Care Provider Level to Improve Inflammatory Breast Cancer Diagnosis and Management. Prev. Med. Rep. 2023, 36, 102519. [Google Scholar] [CrossRef]
- Hamilton, W. Cancer Diagnosis in Primary Care. Br. J. Gen. Pract. 2010, 60, 121–128. [Google Scholar] [CrossRef]
- Al-Azri, M.H. Delay in Cancer Diagnosis: Causes and Possible Solutions. Oman Med. J. 2016, 31, 325–326. [Google Scholar] [CrossRef]
- Sobri, F.; Bachtiar, A.; Panigoro, S.; Ayuningtyas, D.; Gustada, H.; Yuswar, P.; Nur, A.; Putri, R.; Widihidayati, A. Factors Affecting Delayed Presentation and Diagnosis of Breast Cancer in Asian Developing Countries Women: A Systematic Review. Asian Pac. J. Cancer Prev. 2021, 22, 3081–3092. [Google Scholar] [CrossRef]
- O’Donovan, J.; Newcomb, A.; Macrae, M.C.; Vieira, D.; Onyilofor, C.; Ginsburg, O. Community Health Workers and Early Detection of Breast Cancer in Low-Income and Middle-Income Countries: A Systematic Scoping Review of the Literature. BMJ Glob. Health 2020, 5, e002466. [Google Scholar] [CrossRef]
- Nnaji, C.A.; Ezenwankwo, E.F.; Kuodi, P.; Walter, F.M.; Moodley, J. Timeliness of Diagnosis of Breast and Cervical Cancers and Associated Factors in Low-Income and Middle-Income Countries: A Scoping Review. BMJ Open 2022, 12, e057685. [Google Scholar] [CrossRef]
- Baccolini, V.; Isonne, C.; Salerno, C.; Giffi, M.; Migliara, G.; Mazzalai, E.; Turatto, F.; Sinopoli, A.; Rosso, A.; De Vito, C.; et al. The Association between Adherence to Cancer Screening Programs and Health Literacy: A Systematic Review and Meta-Analysis. Prev. Med. 2022, 155, 106927. [Google Scholar] [CrossRef]
- Flytkjær Virgilsen, L.; Møller, H.; Vedsted, P. Cancer Diagnostic Delays and Travel Distance to Health Services: A Nationwide Cohort Study in Denmark. Cancer Epidemiol. 2019, 59, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Benitez Fuentes, J.D.; Morgan, E.; de Luna Aguilar, A.; Mafra, A.; Shah, R.; Giusti, F.; Vignat, J.; Znaor, A.; Musetti, C.; Yip, C.-H.; et al. Global Stage Distribution of Breast Cancer at Diagnosis. JAMA Oncol. 2024, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.G.Q.; Weller, M. Patient Delays and System Delays in Breast Cancer Treatment in Developed and Developing Countries. Cien. Saude Colet. 2015, 20, 3177–3189. [Google Scholar] [CrossRef]
- Regnier Denois, V.; Querre, M.; Chen, L.; Barrault, M.; Chauvin, F. Inequalities and Barriers to the Use of Supportive Care Among Young Breast Cancer Survivors: A Qualitative Understanding. J. Cancer Educ. 2017, 32, 790–798. [Google Scholar] [CrossRef]
- Afaya, A.; Ramazanu, S.; Bolarinwa, O.A.; Yakong, V.N.; Afaya, R.A.; Aboagye, R.G.; Daniels-Donkor, S.S.; Yahaya, A.-R.; Shin, J.; Dzomeku, V.M.; et al. Health System Barriers Influencing Timely Breast Cancer Diagnosis and Treatment among Women in Low and Middle-Income Asian Countries: Evidence from a Mixed-Methods Systematic Review. BMC Health Serv. Res. 2022, 22, 1601. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, R.J. Timing and Delays in Breast Cancer Evaluation and Treatment. Ann. Surg. Oncol. 2018, 25, 2829–2838. [Google Scholar] [CrossRef]
- Fan, R.; Wang, L.; Bu, X.; Wang, W.; Zhu, J. Unmet Supportive Care Needs of Breast Cancer Survivors: A Systematic Scoping Review. BMC Cancer 2023, 23, 587. [Google Scholar] [CrossRef]
- Malapati, S.H.; Hyland, C.J.; Liang, G.; Edelen, M.O.; Fazzalari, A.; Kaur, M.N.; Bain, P.A.; Mody, G.N.; Pusic, A.L. Use of Patient-Reported Outcome Measures after Breast Reconstruction in Low- and Middle-Income Countries: A Scoping Review. J. Patient-Rep. Outcomes 2024, 8, 25. [Google Scholar] [CrossRef]
- Horton, S.; Camacho Rodriguez, R.; Anderson, B.O.; Aung, S.; Awuah, B.; Delgado Pebé, L.; Duggan, C.; Dvaladze, A.; Kumar, S.; Murillo, R.; et al. Health System Strengthening: Integration of Breast Cancer Care for Improved Outcomes. Cancer 2020, 126, 2353–2364. [Google Scholar] [CrossRef]
- Ong, S.K.; Haruyama, R.; Yip, C.H.; Ngan, T.T.; Li, J.; Lai, D.; Zhang, Y.; Yi, S.; Shankar, A.; Suzanna, E.; et al. Feasibility of Monitoring Global Breast Cancer Initiative Framework Key Performance Indicators in 21 Asian National Cancer Centers Alliance Member Countries. EClinicalMedicine 2024, 67, 102365. [Google Scholar] [CrossRef]
- Gbenonsi, G.Y.; Martini, J.; Mahieu, C. An Analytical Framework for Breast Cancer Public Policies in Sub-Saharan Africa: Results from a Comprehensive Literature Review and an Adapted Policy Delphi. BMC Public Health 2024, 24, 1535. [Google Scholar] [CrossRef]
- Kim, J.; Macharia, P.M.; McCormack, V.; Foerster, M.; Galukande, M.; Joffe, M.; Cubasch, H.; Zietsman, A.; Anele, A.; Offiah, S.; et al. Geospatial Disparities in Survival of Patients with Breast Cancer in Sub-Saharan Africa from the African Breast Cancer-Disparities in Outcomes Cohort (ABC-DO): A Prospective Cohort Study. Lancet Glob. Health 2024, 12, e1111–e1119. [Google Scholar] [CrossRef]
- Wöckel, A.; Kurzeder, C.; Geyer, V.; Novasphenny, I.; Wolters, R.; Wischnewsky, M.; Kreienberg, R.; Varga, D. Effects of Guideline Adherence in Primary Breast Cancer–A 5-Year Multi-Center Cohort Study of 3976 Patients. Breast 2010, 19, 120–127. [Google Scholar] [CrossRef]
- Miller, K.; Kreis, I.A.; Gannon, M.R.; Medina, J.; Clements, K.; Horgan, K.; Dodwell, D.; Park, M.H.; Cromwell, D.A. The Association between Guideline Adherence, Age and Overall Survival among Women with Non-Metastatic Breast Cancer: A Systematic Review. Cancer Treat. Rev. 2022, 104, 102353. [Google Scholar] [CrossRef] [PubMed]
- Song, C.V.; Yip, C.-H.; Mohd Taib, N.A.; See, M.H.; Teoh, L.Y.; Monninkhof, E.M.; Saad, M.; Uiterwaal, C.S.P.M.; Bhoo-Pathy, N. Association Between Adherence to Clinical Practice Guidelines for Adjuvant Therapy for Breast Cancer and Survival in a Resource-Limited Setting. JCO Glob. Oncol. 2022, 8, e2100314. [Google Scholar] [CrossRef] [PubMed]
- Manzano, A.; Gralén, K.; Wilking, N.; Hofmarcher, T. Improving Breast Cancer Care in the Middle East and Africa; The Swedish Institute for Health Economics: Lund, Sweden, 2024; Available online: https://ihe.se/app/uploads/2024/04/IHE-REPORT-2024_6_.pdf (accessed on 5 February 2025).
- Davda, J.; Kibet, H.; Achieng, E.; Atundo, L.; Komen, T. Assessing the Acceptability, Reliability, and Validity of the EORTC Quality of Life Questionnaire (QLQ-C30) in Kenyan Cancer Patients: A Cross-Sectional Study. J. Patient-Rep. Outcomes 2021, 5, 4. [Google Scholar] [CrossRef]
- Abate, D.; Aman, M.A.; Nasir, B.B.; Gebremariam, G.T.; Fentie, A.M. Assessment of Quality of Care Using Information on Patient Satisfaction at Adult Oncology Center of Tikur Anbessa Specialized Hospital, Ethiopia: A Cross-Sectional Study. Patient Prefer. Adherence 2020, 14, 847–858. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Country Profiles. Available online: https://www.who.int/teams/noncommunicable-diseases/surveillance/data/cancer-profiles (accessed on 11 December 2025).
- World Health Organization. Model List of Essential Medicines. Available online: https://list.essentialmeds.org/ (accessed on 14 December 2021).
- International Agency for Research on Cancer; World Health Organization. CanScreen5. Available online: https://canscreen5.iarc.fr/ (accessed on 11 December 2025).
- World Health Organization. World Bank Tracking Universal Health Coverage: 2023 Global Monitoring Report; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Bilani, N.; Zabor, E.C.; Elson, L.; Elimimian, E.B.; Nahleh, Z. Breast Cancer in the United States: A Cross-Sectional Overview. J. Cancer Epidemiol. 2020, 2020, 6387378. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Canada. Progress Report 2024; Breast Cancer Canada: Oakville, ON, Canada, 2025. [Google Scholar]
- International Agency for Research on Cancer. National Cancer Registry of Uruguay (Registro Nacional de Cáncer de Uruguay). Available online: https://gicr.iarc.fr/training-center/uruguay-iarc-gicr-collaborating-centre/ (accessed on 11 December 2025).
- Colombia Ministry of Health and Social Protection. Lineamiento para Fortaecimiento de las Acciones para el Control del Cáncer de Mama en el Marco del Plan de Choque [Guideline for the Strengthening of Actions to Control Breast Cancer within the Framework of the Shock Plan]; Colombia Ministry of Health and Social Protection: Bogota, Colombia, 2025.
- Intimayta-Escalante, C. Ethnic inequalities in coverage and use of women’s cancer screening in Peru. BMC Women’s Health 2024, 24, 418. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Câncer; Ministry of Health. Monitoramento Das Ações de Controle Do Câncer de Mama; Informativo Detecção Precoce; Instituto Nacional de Câncer: Rio de Janeiro, Brazil, 2023; Volume 14.
- Campos, A.A.L.; Guerra, M.R.; Fayer, V.A.; Ervilha, R.R.; Cintra, J.R.D.; de Medeiros, I.R.; da Silveira, M.C.; Bustamante-Teixeira, M.T. Time to diagnosis and treatment for breast cancer in public and private health services. Rev. Gaucha Enferm. 2022, 43, e20210103. [Google Scholar] [CrossRef]
- Fonseca, B.d.P.; Albuquerque, P.C.; Saldanha, R.d.F.; Zicker, F. Geographic accessibility to cancer treatment in Brazil: A network analysis. Lancet Reg. Health-Am. 2022, 7, 100153. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Country Fact Sheet: Dominican Republic. CanScreen5. Available online: https://canscreen5.iarc.fr/?page=countryfactsheetbreast&q=DOM (accessed on 11 December 2025).
- Paredes Brito, N.Q. Supervivencia Global y Tasa de Respuesta En Cáncer de Mama HER2 Positivo En Instituto Nacional Del Cáncer Rosa Emilia Sánchez Pérez Tavares En Periodo Agosto 2016–Agosto 2019; Universidad Nacional Pedro Henríquez Ureña: Santo Domingo, Dominican Republic, 2022. [Google Scholar]
- Barrios, C.; Sánchez-Vanegas, G.; Villarreal-Garza, C.; Ossa, A.; Lombana, M.A.; Monterrosa-Blanco, A.; Ferrigno, A.S.; Castro, C.A. Barriers and facilitators to provide multidisciplinary care for breast cancer patients in five Latin American countries: A descriptive-interpretative qualitative study. Lancet Reg. Health-Am. 2022, 11, 100254. [Google Scholar] [CrossRef]
- Chavarri-Guerra, Y.; Louis, J.S.; Liedke, P.E.; Symecko, H.; Villarreal-Garza, C.; Mohar, A.; Finkelstein, D.M.; Goss, P.E. Access to care issues adversely affect breast cancer patients in Mexico: Oncologists’ perspective. BMC Cancer 2014, 14, 658. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Country Fact Sheet: Mexico. CanScreen5. 2025. Available online: https://canscreen5.iarc.fr/?page=countryfactsheet&q=MEX (accessed on 11 December 2025).
- International Agency for Research on Cancer. Country Fact Sheet: Cuba. CanScreen5. Available online: https://canscreen5.iarc.fr/?page=countryfactsheetbreast&q=CUB (accessed on 11 December 2025).
- Pan American Health Organization. Age Standardized Cancer Mortality Trends (2001–2010) 6—Cuba; Pan American Health Organization: Washington, DC, USA, 2013. [Google Scholar]
- Flood, D.; Chary, A.; Austad, K.; Coj, M.; Lopez, W.; Rohloff, P. Patient Navigation and Access to Cancer Care in Guatemala. J. Glob. Oncol. 2018, 4, 1–3. [Google Scholar] [CrossRef]
- Mosquera, I.; Ilbawi, A.; Muwonge, R.; Basu, P.; Carvalho, A.L. Cancer burden and status of cancer control measures in fragile states: A comparative analysis of 31 countries. Lancet Glob. Health 2022, 10, e1443–e1452. [Google Scholar] [CrossRef]
- Ragozzino, M.R. How the Crisis Endangers Breast Cancer Patients. Available online: https://www.hrw.org/news/2021/11/03/how-crisis-endangers-breast-cancer-patients (accessed on 11 December 2025).
- Stuart, G.W.; Chamberlain, J.A.; Marvelde, L.T. The contribution of prognostic factors to socio-demographic inequalities in breast cancer survival in Victoria, Australia. Cancer Med. 2023, 12, 15371–15383. [Google Scholar] [CrossRef]
- Ellison-Loschmann, L.; Firestone, R.; Aquilina, L.; McKenzie, F.; Gray, M.; Jeffreys, M. Barriers to and delays in accessing breast cancer care among New Zealand women: Disparities by ethnicity. BMC Health Serv. Res. 2015, 15, 394. [Google Scholar] [CrossRef]
- Boyle, L.; Lawrenson, R.; Nosa, V.; Campbell, I.; Tin, S.T. Ethnic inequities in use of breast conserving surgery and radiation therapy in Aotearoa/New Zealand: Which factors contribute? Breast Cancer Res. Treat. 2024, 205, 641–653. [Google Scholar] [CrossRef]
- Abubakar, A.K.; Kaneda, Y.; Ozaki, A.; Saito, H.; Murakami, M.; Hori, D.; Gonda, K.; Tsubokura, M.; Tabuchi, T. Two-Year-Span Breast Cancer Screening Uptake in Japan after the COVID-19 Pandemic and Its Association with the COVID-19 Vaccination. Cancers 2024, 16, 1783. [Google Scholar] [CrossRef]
- Saeki, S.; Iwatani, T.; Kitano, A.; Sakurai, N.; Tanabe, Y.; Yamauchi, C.; Igarashi, A.; Kajimoto, Y.; Kuba, S.; Hara, F.; et al. Factors associated with financial toxicity in patients with breast cancer in Japan: A comparison of patient and physician perspectives. Breast Cancer 2023, 30, 820–830. [Google Scholar] [CrossRef]
- Nagahashi, M.; Kumamaru, H.; Kinukawa, N.; Iwamoto, T.; Kawashima, M.; Kinoshita, T.; Konishi, T.; Sagara, Y.; Sasada, S.; Saji, S.; et al. Breast cancer statistics for Japan in 2022: Annual report of the national clinical database-breast cancer registry—Clinical implications including chemosensitivity of breast cancer with low estrogen receptor expression. Breast Cancer 2025, 32, 217–226. [Google Scholar] [CrossRef]
- Malaysia Ministry of Health. Trastuzumab as an Adjuvant Therapy for Early Breast Cancer and Economic Evaluation. Available online: https://www.inahta.org/upload/2017/17034_Trastuzumab%20as%20an%20Adjuvant%20Therapy%20for%20Early%20Breast%20Cancer.pdf (accessed on 11 December 2025).
- Sruamsiri, R.; Ross-Degnan, D.; Lu, C.Y.; Chaiyakunapruk, N.; Wagner, A.K. Policies and Programs to Facilitate Access to Targeted Cancer Therapies in Thailand. PLoS ONE 2015, 10, e0119945. [Google Scholar] [CrossRef]
- Poum, A.; Promthet, S.; Duffy, S.W.; Parkin, D.M. Factors Associated With Delayed Diagnosis of Breast Cancer in Northeast Thailand. J. Epidemiol. 2014, 24, 102–108. [Google Scholar] [CrossRef]
- Mathur, P.; Sathishkumar, K.; Chaturvedi, M.; Das, P.; Sudarshan, K.L.; Santhappan, S.; Nallasamy, V.; John, A.; Narasimhan, S.; Roselind, F.S.; et al. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob. Oncol. 2020, 6, 1063–1075. [Google Scholar] [CrossRef]
- Mehrotra, R.; Yadav, K. Breast cancer in India: Present scenario and the challenges ahead. World J. Clin. Oncol. 2022, 13, 209–218. [Google Scholar] [CrossRef]
- Hutajulu, S.H.; Prabandari, Y.S.; Bintoro, B.S.; Wiranata, J.A.; Widiastuti, M.; Suryani, N.D.; Saptari, R.G.; Taroeno-Hariadi, K.W.; Kurnianda, J.; Purwanto, I.; et al. Delays in the presentation and diagnosis of women with breast cancer in Yogyakarta, Indonesia: A retrospective observational study. PLoS ONE 2022, 17, e0262468. [Google Scholar] [CrossRef]
- Khoirunnisa, S.M. Burden, Challenges, and Future Directions for Breast Cancer Treatment in Indonesia. Ph.D. Thesis, University of Groningen, Netherlands, February 2025. [Google Scholar] [CrossRef]
- Peiris, G.S.; Pawiro, S.A.; Kasim, M.F.; Sheehy, S.L. Failure modes and downtime of radiotherapy LINACs and multileaf collimators in Indonesia. J. Appl. Clin. Med Phys. 2022, 24, e13756. [Google Scholar] [CrossRef]
- Omidi, Z.; Koosha, M.; Nazeri, N.; Khosravi, N.; Zolfaghari, S.; Haghighat, S. Status of breast cancer screening strategies and indicators in Iran. J. Res. Med Sci. 2022, 27, 21. [Google Scholar] [CrossRef]
- Goudarzi, Z.; Nouhi, M.; Heydari, M.; Bijlmakers, L. Availability, affordability and health insurance coverage of breast cancer services in Iran—An analysis based on the Universal Health Coverage-Service Planning Delivery and Implementation tool. J. Cancer Policy 2025, 44, 100571. [Google Scholar] [CrossRef]
- Akbari, M.E.; Akbari, A.; Khayamzadeh, M.; Salmanian, R.; Akbari, M. Ten-Year Survival of Breast Cancer in Iran: A National Study (Retrospective Cohort Study). Breast Care 2022, 18, 12–21. [Google Scholar] [CrossRef]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Austria; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- Ding, L.; Jidkova, S.; Greuter, M.J.W.; Van Herck, K.; Goossens, M.; Martens, P.; de Bock, G.H.; Van Hal, G. Coverage determinants of breast cancer screening in Flanders: An evaluation of the past decade. Int. J. Equity Health 2020, 19, 212. [Google Scholar] [CrossRef]
- Goossens, M.M.; Kellen, E.; Broeders, M.J.M.; Vandemaele, E.; Jacobs, B.; Martens, P. The effect of a pre-scheduled appointment on attendance in a population-based mammography screening programme. Eur. J. Public Health 2023, 33, 1122–1127. [Google Scholar] [CrossRef]
- Lynge, E.; Bak, M.; von Euler-Chelpin, M.; Kroman, N.; Lernevall, A.; Mogensen, N.B.; Schwartz, W.; Wronecki, A.J.; Vejborg, I. Outcome of breast cancer screening in Denmark. BMC Cancer 2017, 17, 897. [Google Scholar] [CrossRef]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Ireland; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Norway; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- Skjerven, H.K.; Trewin-Nybråten, C.B.; Kjersti, K. The Norwegian Breast Cancer Registry (NBCR): A clinical register that monitors surgical care with the intention to increase the quality of treatment given to breast cancer patients in Norway. Nor. Epidemiol. 2022, 30, 41–46. [Google Scholar] [CrossRef]
- Hofmarcher, T.; Berchet, C.; Dedet, G. Access to Oncology Medicines in EU and OECD Countries; OECD Health Working Papers No. 170; OECD: Paris, France, 2024. [Google Scholar]
- Suter, F.; Wanner, M.; Wicki, A.; Korol, D.; Rohrmann, S. Effect of the COVID-19 pandemic and lockdown on cancer stage distribution and time to treatment initiation using cancer registry data of the Swiss cantons of Zurich and Zug from 2018 to 2021. J. Cancer Res. Clin. Oncol. 2025, 151, 88. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Khan, S.; Marder, D.; Zwahlen, D.; Bodis, S. Radiotherapy infrastructure and human resources in Switzerland. Strahlenther. Onkol. 2016, 192, 599–608. [Google Scholar] [CrossRef]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: France; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Germany; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Italy; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- John, S.; Broggio, J. Cancer Survival in England-Adults Diagnosed. 2019. Available online: https://www.nuffieldtrust.org.uk/resource/cancer-survival-rates (accessed on 19 October 2020).
- Nuffield Trust Cancer Screening. Quality Watch. Available online: https://www.nuffieldtrust.org.uk/resource/breast-and-cervical-cancer-screening (accessed on 11 December 2025).
- Nuffield Trust Cancer Waiting Times. Quality Watch. Available online: https://www.nuffieldtrust.org.uk/resource/cancer-waiting-time-targets (accessed on 11 December 2025).
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Netherlands; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Portugal; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Spain; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Sweden; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Croatia; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- Todorovic, J.; Stamenkovic, Z.; Stevanovic, A.; Terzic, N.; Kissimova-Skarbek, K.; Tozija, F.; Mechili, E.A.; Devleesschauwer, B.; Terzic-Supic, Z.; Vasic, M.; et al. The burden of breast, cervical, and colon and rectum cancer in the Balkan countries, 1990–2019 and forecast to 2030. Arch. Public Heal. 2023, 81, 156. [Google Scholar] [CrossRef]
- Lokvančić, H. HER2 positive breast cancer and its treatment with trastuzumab, where are we now? Bioeng. Stud. 2022, 3, 35–43. [Google Scholar] [CrossRef]
- Bešlija, S.; Gojković, Z.; Cerić, T.; Abazović, A.M.; Marijanović, I.; Vranić, S.; Mustedanagić–Mujanović, J.; Skenderi, F.; Rakita, I.; Guzijan, A.; et al. 2020 consensus guideline for optimal approach to the diagnosis and treatment of HER2-positive breast cancer in Bosnia and Herzegovina. Bosn. J. Basic Med Sci. 2020, 21, 120–135. [Google Scholar] [CrossRef]
- Hadžikadić-Gušić, L.; Cerić, T.; Marijanović, I.; Iljazović, E.; Koprić, D.; Zorlak, A.; Tanović, M.; Mekić-Abazović, A.; Šišić, I.; Delić, U.; et al. Guidelines for breast cancer management in Bosnia and Herzegovina. Biomol. Biomed. 2023, 23, 2–14. [Google Scholar] [CrossRef]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Greece; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Hungary; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- Barchuk, A.; Belyaev, A.; Gretsova, O.; Tursun-Zade, R.; Moshina, N.; Znaor, A. History and current status of cancer registration in Russia. Cancer Epidemiol. 2021, 73, 101963. [Google Scholar] [CrossRef]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Bulgaria; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- Jasiura, A.; Dera, I.; Szlachcic, K.; Gorzel, M.; Zmonarska, J. Breast cancer screening programmes in selected European countries and Poland. J. Educ. Health Sport 2021, 11, 11–21. [Google Scholar] [CrossRef]
- European Cancer Inequalities Registry. OECD Country Cancer Profile 2025: Poland; European Cancer Inequalities Registry: Paris, France, 2025. [Google Scholar]
- International Agency for Research on Cancer. Current Status and Future Directions of Breast and Cervical Cancer Prevention and Early Detection in Belarus; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Mandrik, O.; Yaumenenka, A.; Herrero, R.; Jonker, M.F. Population preferences for breast cancer screening policies: Discrete choice experiment in Belarus. PLoS ONE 2019, 14, e0224667. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Belarus to Strengthen Cancer Services, Building on IAEA ImPACT Review. Available online: https://www.iaea.org/newscenter/news/belarus-to-strengthen-cancer-services-building-on-iaea-impact-review (accessed on 11 December 2025).
- Selmani, E.; Hoxha, I.; Tril, O.; Khan, O.; Hrynkiv, A.; Nogueira, L.; Pyle, D.; Chamberlin, M. Fighting Cancer in Ukraine at Times of War. Hematol. Clin. N. Am. 2023, 38, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, T.; Moonesar, I.A. Financial Restrictions Limit Early Breast Cancer Screening: The Case of Jordan. J. Oncol. 2022, 2, 1037. [Google Scholar]
- Helalah, A.; Munir, A.; Alshraideh, A.H.; Al-Hanaqtah, M.; Da’Na, M.; Al-Omari, A.; Mubaidin, R. Delay in Presentation, Diagnosis, and Treatment for Breast Cancer Patients in Jordan. Breast J. 2015, 22, 213–217. [Google Scholar] [CrossRef]
- Salem, A.; Al-Ramahi, L.; Alodeh, S.; Al-Sarayrah, W.; Hussein, A.M.; Al-Qudah, M.; Abdel-Razeq, H.; Bashaireh, K.; Alsmarat, F. Analysis of the Demand and Supply for Oncology Workforce in Jordan: Current Status and Future Projections. JCO Glob. Oncol. 2025, 11, e2400638. [Google Scholar] [CrossRef]
- Abdel-Razeq, H.; Al-Ibraheem, A.; Al-Rabi, K.; Shamiah, O.; Al-Husaini, M.; Mansour, A. Cancer Care in Resource-Limited Countries: Jordan as an Example. JCO Glob. Oncol. 2024, 10, e2400237. [Google Scholar] [CrossRef]
- The Swedish Institute for Health Economics. Country Card Algeria: Improving Breast Cancer in the MEA Region; The Swedish Institute for Health Economics: Lund, Sweden, 2024. [Google Scholar]
- Zhao, J.; Yabroff, K.R. High out-of-pocket spending and financial hardship at the end of life among cancer survivors and their families. Isr. J. Health Policy Res. 2023, 12, 24. [Google Scholar] [CrossRef]
- Bentur, N.; Emanuel, L.L.; Cherney, N. Progress in palliative care in Israel: Comparative mapping and next steps. Isr. J. Health Policy Res. 2012, 1, 9. [Google Scholar] [CrossRef]
- Yad L’Olim. Palliative & Hospice Care. Available online: https://www.yadlolim.org/healthcare/palliative-hospice-care (accessed on 11 December 2025).
- The Swedish Institute for Health Economics. Country Card Turkey Improving Breast Cancer in the MEA Region; The Swedish Institute for Health Economics: Lund, Sweden, 2024. [Google Scholar]
- Al-Shahri, M.Z. Cancer pain: Progress and ongoing issues in Saudi Arabia. Pain Res. Manag. 2009, 14, 359–360. [Google Scholar]
- Council of Health Insurance. Health Insurance Companies Are Obliged to Cover Breast Cancer Treatment and Reconstructive Surgeries. Available online: https://www.chi.gov.sa/en/MediaCenter/News/pages/news-01-10-2019.aspx?# (accessed on 11 December 2025).
- Gray, A.; Ezzat, A. Palliative Care for Patients with Advanced Cancer. J. Fam. Community Med. 1997, 4, 41–46. [Google Scholar] [CrossRef]
- Almobarak, F. Unlocking compassion: Expanding access to palliative care in Saudi Arabia. Palliat. Care Soc. Pract. 2024, 18, 26323524241290828. [Google Scholar] [CrossRef]
- Alessy, S.A.; Al-Zahrani, A.; Alhomoud, S.; Alaskar, A.; Haoudi, A.; Alkheilewi, M.A.; Alhamali, M.; Alsharm, A.A.; Asiri, M.; Alqahtani, S.A. Towards a comprehensive cancer control policy in Saudi Arabia. Lancet Oncol. 2025, 26, e360–e368. [Google Scholar] [CrossRef]
- The Swedish Institute for Health Economics. Country Card Saudi Arabia: Improving Breast Cancer in the MEA Region; The Swedish Institute for Health Economics: Lund, Sweden, 2024. [Google Scholar]
- The Swedish Institute for Health Economics. Country Card Egypt Improving Breast Cancer in the MEA Region; The Swedish Institute for Health Economics: Lund, Sweden, 2024. [Google Scholar]
- Elias-Rizk, T.; Issa, E.; Ammanouil, E.; Khalil, M.A.; Salameh, P.; Abi-Gerges, A. Breast cancer screening in Lebanon: Understanding knowledge, attitudes and barriers. Clin. Epidemiology Glob. Health 2024, 29, 101733. [Google Scholar] [CrossRef]
- Issa, E.; Lahoud, R.; Abi-Gerges, A.; Salameh, P.; Elias-Rizk, T. Breast cancer screening practices during a multifaceted crisis: Data from Lebanon. PLoS ONE 2025, 20, e0325604. [Google Scholar] [CrossRef]
- Mohty, R.; Tfayli, A. General Oncology Care in Lebanon. In Cancer in the Arab World; Springer: Singapore, 2022; pp. 115–132. [Google Scholar]
- The Swedish Institute for Health Economics. Country Card Morocco Improving Breast Cancer in the MEA Region; The Swedish Institute for Health Economics: Lund, Sweden, 2024. [Google Scholar]
- Balhi, S.; Khiari, H.; Hsairi, M. Factors Associated with Diagnostic Delays among Tunisian Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2023, 24, 471–477. [Google Scholar] [CrossRef]
- Jemaà, M. Cancer Diagnosis In Tunisian Public Structures: Too Little, Too Late. Eurasian J. Med. Adv. 2023, 3, 160–163. [Google Scholar]
- Owoko, L. WHO Ranks Kenya as Africa’s Top in Breast Cancer Control. Available online: https://www.businessdailyafrica.com/bd/corporate/health/who-ranks-kenya-as-africa-s-top-in-breast-cancer-control-4940404 (accessed on 11 December 2025).
- Gakunga, R.; Kinyanjui, A.; Ali, Z.; Ochieng’, E.; Gikaara, N.; Maluni, F.; Wata, D.; Kyeng’, M.; Korir, A.; Subramanian, S. Identifying Barriers and Facilitators to Breast Cancer Early Detection and Subsequent Treatment Engagement in Kenya: A Qualitative Approach. Oncologist 2019, 24, 1549–1556. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Breast Cancer Control Capacities in the WHO African Region; World Health Organization: Brazzaville, Congo, 2022. [Google Scholar]
- The Swedish Institute for Health Economics. Country Card South Africa Improving Breast Cancer in the MEA Region; The Swedish Institute for Health Economics: Lund, Sweden, 2024. [Google Scholar]
- International Atomic Energy Agency. Empowering Guinea: The IAEA Provides Guidance on Cancer Control Measures to One of Its Newest Member States. Available online: https://www.iaea.org/newscenter/news/empowering-guinea-the-iaea-provides-guidance-on-cancer-control-measures-to-one-of-its-newest-member-states (accessed on 11 December 2025).
- Shakor, J.K. Assessment of the Iraqi Breast Cancer Early Detection and Downstaging Program: Mammography Cancer Detection Rate. Passer J. Basic Appl. Sci. 2023, 5, 272–277. [Google Scholar] [CrossRef]
- National Institute for Cancer Research and Treatment. National Strategic Cancer Control Plan (2023–2027); National Institute for Cancer Research and Treatment: Abuja, Nigeria, 2023.
- Nahhat, F.; Doyya, M.; Zabad, K.; Laban, T.A.; Najjar, H.; Saifo, M.; Badin, F. Breast cancer quality of care in Syria: Screening, diagnosis, and staging. BMC Cancer 2023, 23, 1234. [Google Scholar] [CrossRef]
| Region | Incidence Absolute Value | Incidence Age Standardized Rate Per 100,000 | Mortality Absolute Value | Mortality Age Standardized Rate Per 100,000 | 5-Year Prevalence Absolute Value |
|---|---|---|---|---|---|
| Global | 2,296,840 | 46.8 | 666,103 | 12.7 | 8,178,393 |
| Asia | 985,817 | 34.3 | 315,309 | 10.5 | 3,197,043 |
| Europe | 557,532 | 75.6 | 144,439 | 14.6 | 2,296,495 |
| North America | 306,307 | 95.1 | 91,252 | 12.3 | 1,332,343 |
| Latin America and the Caribbean | 220,124 | 52.0 | 59,876 | 13.2 | 725,017 |
| Africa | 198,553 | 40.5 | 49,744 | 19.2 | 507,659 |
| Oceania | 28,507 | 91.5 | 5483 | 15.4 | 119,836 |
| Sustainable Development Goals (SDGs) and Targets | Goal/Target |
|---|---|
| SDG 3.4 | By 2030, reduce by one third premature mortality from non-communicable diseases through prevention and treatment and promote mental health and well-being |
| SDG 3.8 | Achieve universal health coverage, including financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all |
| SDG 5 | Achieve gender equality and empower all women and girls |
| SDG 10 | Reduce inequality within and among countries |
| Dimension Identification, Dimension, and Goals | Target/ Indicator Identification | Target/Indicator Focus |
|---|---|---|
| A: Early Breast Cancer Detection Promote early detection of breast cancer | A.1 | Existence of national programs, policies, or frameworks for early breast cancer detection. |
| A.1.1 | Existence of national program, policy, or framework. | |
| A.1.2 | Inclusion of breast cancer awareness and education programs for the public and healthcare workers in national health-related frameworks. | |
| A.2 | Availability of and access to breast cancer early detection programs and services. | |
| A.2.1 | Execution of breast cancer early detection programs and services. | |
| A.2.2 | QUANTITATIVE INDICATOR: Proportion of women at elevated risk of breast cancer screened at least once every two years. | |
| A.3 | Ensure that at least 60% of invasive breast cancers are diagnosed at stage I or II. | |
| A.3.1 | QUANTITATIVE INDICATOR: Proportion of invasive cancers diagnosed at stage I or II according to (Tumor, Nodes, and Metastasis) TNM anatomic and/or pathological staging. | |
| B: Timely Breast Cancer Diagnosis Ensure timely access to appropriate breast cancer diagnosis | B.1 | Access to diagnostic services. |
| B.1.1 | Existence of a national policy or framework for breast cancer diagnostic services. | |
| B.2 | Ensure diagnosis completion within two months from first access/presentation due to a suspicious finding. | |
| B.2.1 | QUANTITATIVE INDICATOR: Proportion of patients with complete diagnosis and appropriate staging within two months from first access/presentation due to a suspicious finding. | |
| C: Comprehensive Breast Cancer Management Guarantee timely access to comprehensive breast cancer treatment and care for all patients at all stages | C.1 | Access to breast cancer care and management. |
| C.1.1 | Existence of a national policy or framework for access to breast cancer care. | |
| C.1.2 | QUANTITATIVE INDICATOR: Proportion of patients with timely treatment initiation. | |
| C.1.3 | QUANTITATIVE INDICATOR: Proportion of triple-negative breast cancer, HER2+, and (Hormone Receptor positive / HER2 negative) HR+/HER- early breast cancer patients who receive adequate treatment. | |
| C.1.4 | QUANTITATIVE INDICATOR: Proportion of patients with hormone receptor-positive invasive breast cancer who receive adequate treatment. | |
| C.1.5 | QUANTITATIVE INDICATOR: Proportion of breast cancer patients that receive adequate supportive services. | |
| C.1.6 | Incorporation of patient perspective in service quality assessment protocols for breast cancer. | |
| C.1.7 | Existence of a national program, policy, or framework for survivorship care plan implementation. | |
| C.2 | Ensure treatment completion for more than 80% of breast cancer patients. | |
| C.2.1 | QUANTITATIVE INDICATOR: Proportion of patients who complete their recommended treatment out of the total number of patients treated. | |
| D: Strong and Resilient Healthcare Systems Strengthen overall health system capacity for health promotion, and breast cancer diagnosis, treatment, and care | D.1 | Strengthen healthcare system’s infrastructure, capability, capacity, knowledge, and resources. |
| D.1.1 | Availability of sustainable sources of funding for breast cancer. | |
| D.1.2 | QUANTITATIVE INDICATOR: Number of breast cancer-specialized healthcare professionals per 10,000 cancer patients. | |
| D.1.3 | QUANTITATIVE INDICATOR: Number of specialized hospital units or departments that provide multidisciplinary breast cancer care per 10,000 cancer patients. | |
| D.2 | Availability of data regarding breast cancer. | |
| D.2.1 | Existence of population-wide data through national or regional cancer registries. | |
| D.2.2 | QUANTITATIVE INDICATOR: Yearly breast cancer mortality and/or 5-year survival. | |
| D.3 | Availability and application of clinical practice guidelines and coordination of care mechanisms. | |
| D.3.1 | Existence of a framework for clinical practice guidelines adoption, dissemination, and adherence. | |
| D.3.2 | Existence of a well-defined service integration/patient navigation mechanism. | |
| D.3.3 | Existence of a framework to ensure patient engagement in healthcare decision-making and health service planning and design. |
| I. Low level of achievement | II. Modest level of achievement |
|
|
| III. Moderate level of achievement | IV. Outstanding level of achievement |
|
|
| I. Low level of achievement | II. Modest level of achievement |
|
|
| III. Moderate level of achievement | IV. Outstanding level of achievement |
|
|
| I. Low level of achievement | II. Modest level of achievement |
|
|
| III. Moderate level of achievement | IV. Outstanding level of achievement |
|
|
| I. Low level of achievement | II. Modest level of achievement |
|
|
| III. Moderate level of achievement | IV. Outstanding level of achievement |
|
|
| Policymakers |
| Stakeholders in a country’s legislative and executive branches, including agencies and individuals with formal authority to develop, enact, enforce, and oversee laws, regulations, and national policies on health, including breast cancer. This category covers ministries of health, national cancer control offices, parliamentarians, regulatory authorities, and other government decision-makers at national or subnational levels. |
| Multisectoral Stakeholders |
| Non-governmental actors at national or subnational levels whose expertise, influence, and resources contribute to the design, implementation, and uptake of breast cancer strategies. This group includes scientific and medical societies, civil society and patient advocacy groups, trade associations, academia and research institutions, think tanks, professional networks, health providers, and philanthropic organizations. They are directly impacted by breast cancer policies but operate outside formal policymaking structures and supranational governance bodies. |
| International Community |
| Supranational and intergovernmental organizations, their agencies, and funds that influence or coordinate health policy globally or regionally. This includes, among others, the United Nations system, the WHO, the World Bank, the Organisation for Economic Co-operation and Development, and similar bodies, as well as their specialized agencies, technical programs, and financing mechanisms. |
| I. Low level of achievement | II. Modest level of achievement |
|
|
| III. Moderate level of achievement | IV. Outstanding level of achievement |
|
|
| I. Low level of achievement | II. Modest level of achievement |
|
|
| III. Moderate level of achievement | IV. Outstanding level of achievement |
|
|
| I. Low level of achievement | II. Modest level of achievement |
|
|
| III. Moderate level of achievement | IV. Outstanding level of achievement |
|
|
| I. Low level of achievement | II. Modest level of achievement |
|
|
| III. Moderate level of achievement | IV. Outstanding level of achievement |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Caleffi, M.; Ajango, M.; Al-Awadhi, A.M.; Fairley, R.; Feigl, A.B.; González, A.R.; Harmer, V.; Nekkalapudi, N.; Saraki, T.; Smart, V.W.; et al. Improving Breast Cancer Outcomes Through Quality Care: Call to Action for the Implementation of the Breast Cancer Care Quality Index (BCCQI). Int. J. Environ. Res. Public Health 2026, 23, 207. https://doi.org/10.3390/ijerph23020207
Caleffi M, Ajango M, Al-Awadhi AM, Fairley R, Feigl AB, González AR, Harmer V, Nekkalapudi N, Saraki T, Smart VW, et al. Improving Breast Cancer Outcomes Through Quality Care: Call to Action for the Implementation of the Breast Cancer Care Quality Index (BCCQI). International Journal of Environmental Research and Public Health. 2026; 23(2):207. https://doi.org/10.3390/ijerph23020207
Chicago/Turabian StyleCaleffi, Maira, Mary Ajango, Aydah M. Al-Awadhi, Ricki Fairley, Andrea B. Feigl, Ana Rita González, Victoria Harmer, Naveena Nekkalapudi, Toyin Saraki, Victoria Wolodzko Smart, and et al. 2026. "Improving Breast Cancer Outcomes Through Quality Care: Call to Action for the Implementation of the Breast Cancer Care Quality Index (BCCQI)" International Journal of Environmental Research and Public Health 23, no. 2: 207. https://doi.org/10.3390/ijerph23020207
APA StyleCaleffi, M., Ajango, M., Al-Awadhi, A. M., Fairley, R., Feigl, A. B., González, A. R., Harmer, V., Nekkalapudi, N., Saraki, T., Smart, V. W., Fernandez-Cerdeño, A., Rocha, J. V., Lucibello, I., & Srivastava, N. (2026). Improving Breast Cancer Outcomes Through Quality Care: Call to Action for the Implementation of the Breast Cancer Care Quality Index (BCCQI). International Journal of Environmental Research and Public Health, 23(2), 207. https://doi.org/10.3390/ijerph23020207






